��Ŀ����
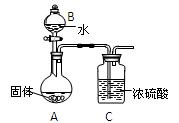
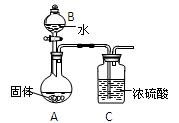
ʵ��С��ͬѧ̽��ij��ʯ��������FeCO3��SiO2��Al2O3�е�һ�ֻ�����ɣ�̽��������ͼ��ʾ��
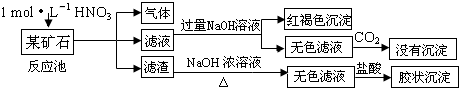
��ش��������⣺
��1��Fe�����ڱ��е�λ���� ��
��2������˵����ȷ���ǣ�ѡ���ţ� ��
a�����ԣ�H2CO3��H2SiO3 b��ԭ�Ӱ뾶��O��C��Si��Al
c���ȶ��ԣ�H2O��CH4��SiH4 d�����Ӱ뾶��O2-��Al3+
��3���ÿ�ʯ������� ��������NaOH��Һ��Ӧ�����ӷ���ʽ�� ��
��4���ÿ�ʯ��1mol?L-1 HNO3��Ӧ�����ӷ���ʽ�� ��
��5����ҵ����������ʵ��ԭ�������ÿ�ʯ������Ӧ���ݳ���������һ������O2���ѭ��ͨ�뷴Ӧ���У�Ŀ���� ���������ÿ�ʯ2.36��103 kg���õ�����1.2��103 kg��������������Ҫ1mol?L-1 HNO3�����Ϊ L��

��ش��������⣺
��1��Fe�����ڱ��е�λ����
��2������˵����ȷ���ǣ�ѡ���ţ�
a�����ԣ�H2CO3��H2SiO3 b��ԭ�Ӱ뾶��O��C��Si��Al
c���ȶ��ԣ�H2O��CH4��SiH4 d�����Ӱ뾶��O2-��Al3+
��3���ÿ�ʯ�������
��4���ÿ�ʯ��1mol?L-1 HNO3��Ӧ�����ӷ���ʽ��
��5����ҵ����������ʵ��ԭ�������ÿ�ʯ������Ӧ���ݳ���������һ������O2���ѭ��ͨ�뷴Ӧ���У�Ŀ����
��������1����������ԭ�Ӻ�������Ų�ȷ�������ڱ��е�λ�ã�
��2��a��Ԫ�صķǽ�����Խǿ����Ӧ������������ˮ���������Խǿ��
b�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ���Ӳ���ͬ��ԭ�Ӻ˵����Խ�뾶ԽС��
c��Ԫ�صķǽ�����Խǿ����Ӧ���⻯��Խ�ȶ���
d���������Ӻ�������Ų���ͬ���˵����Խ�����Ӱ뾶ԽС��
��3����ʯ�������������壬��˵������FeCO3���������NaoH��Һ��ͨ�����������̼û�г������ɣ�˵��û��Al2O3��
�����ܽ������������ɽ�״������˵������SiO2��
��4����ʯ�к���FeCO3������HNO3����������ԭ��Ӧ��
��5�������ÿ�ʯ���ݳ�������ΪNO������������Ӧ�������ᣬ��ѭ�����ã���Ϸ�Ӧ�ķ���ʽ���㣮
��2��a��Ԫ�صķǽ�����Խǿ����Ӧ������������ˮ���������Խǿ��
b�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ���Ӳ���ͬ��ԭ�Ӻ˵����Խ�뾶ԽС��
c��Ԫ�صķǽ�����Խǿ����Ӧ���⻯��Խ�ȶ���
d���������Ӻ�������Ų���ͬ���˵����Խ�����Ӱ뾶ԽС��
��3����ʯ�������������壬��˵������FeCO3���������NaoH��Һ��ͨ�����������̼û�г������ɣ�˵��û��Al2O3��
�����ܽ������������ɽ�״������˵������SiO2��
��4����ʯ�к���FeCO3������HNO3����������ԭ��Ӧ��
��5�������ÿ�ʯ���ݳ�������ΪNO������������Ӧ�������ᣬ��ѭ�����ã���Ϸ�Ӧ�ķ���ʽ���㣮
����⣺��1����Ԫ�������ڱ�����26��Ԫ�أ���Ԫ��λ�ڵ������ڢ��壬
�ʴ�Ϊ���������ڢ���
��2��a���ǽ����ԣ�C��Si��Ԫ�صķǽ�����Խǿ����Ӧ������������ˮ���������Խǿ����a��ȷ��
b��ͬ����Ԫ�ش�����ԭ�Ӱ뾶��С��O��C��Si��Al��ͬ����Ԫ�ش��ϵ���ԭ�Ӱ뾶��������C��Si����b��ȷ��
c���ǽ�����O��C��Si��Ԫ�صķǽ�����Խǿ����Ӧ���⻯��Խ�ȶ�����c��ȷ��
d���������Ӻ�������Ų���ͬ���˵����Խ�����Ӱ뾶ԽС����O2-��Al3+����d����
�ʴ�Ϊ��abc��
��3�����ڿ�ʯ����������Ӧ�������壬��˵������FeCO3���������NaoH��Һ��ͨ�����������̼û�г������ɣ�˵��û��Al2O3�������ܽ������������ɽ�״������˵������SiO2��������NaOH��Һ��Ӧ�����ӷ���ʽ��SiO2+2OH-=SiO32-+H2O��
�ʴ�Ϊ��FeCO3��SiO2��SiO2+2OH-=SiO32-+H2O��
��4�����ڿ�ʯ����FeCO3���������ӱ�����������ͬʱ���ᱻ��ԭ��һ����������Ӧ�����ӷ���ʽΪ��3FeCO3+10H++NO3-=3Fe3++3CO2��+NO��+5H2O��
�ʴ�Ϊ��3FeCO3+10H++NO3-=3Fe3++3CO2��+NO��+5H2O��
��5������NO���ж����壬���������ŷţ�������������Ŀ����NOѭ��ʹ���ܼ��ٻ�����Ⱦ����NO��H2O��O2��Ӧ���ֵõ��������ԭ�������ʣ��������Ϸ�����֪���ÿ�ʯ�к���̼�������Ͷ������裬����̼��������������2.36��103 kg-1.2��103 kg=1.16��103kg�����ʵ�����10000mol�������3FeCO3+10H++NO3-=3Fe3++3CO2��+NO��+5H2O�еķ���ʽ��֪��������������ʵ����ǣ�
mol������������������Ҫ1molL-1 HNO3�����Ϊ3.0��104L��
�ʴ�Ϊ��NOѭ��ʹ���ܼ��ٻ�����Ⱦ��NO��H2O��O2��Ӧ���ֵõ��������ԭ�������ʣ�3��104��
�ʴ�Ϊ���������ڢ���
��2��a���ǽ����ԣ�C��Si��Ԫ�صķǽ�����Խǿ����Ӧ������������ˮ���������Խǿ����a��ȷ��
b��ͬ����Ԫ�ش�����ԭ�Ӱ뾶��С��O��C��Si��Al��ͬ����Ԫ�ش��ϵ���ԭ�Ӱ뾶��������C��Si����b��ȷ��
c���ǽ�����O��C��Si��Ԫ�صķǽ�����Խǿ����Ӧ���⻯��Խ�ȶ�����c��ȷ��
d���������Ӻ�������Ų���ͬ���˵����Խ�����Ӱ뾶ԽС����O2-��Al3+����d����
�ʴ�Ϊ��abc��
��3�����ڿ�ʯ����������Ӧ�������壬��˵������FeCO3���������NaoH��Һ��ͨ�����������̼û�г������ɣ�˵��û��Al2O3�������ܽ������������ɽ�״������˵������SiO2��������NaOH��Һ��Ӧ�����ӷ���ʽ��SiO2+2OH-=SiO32-+H2O��
�ʴ�Ϊ��FeCO3��SiO2��SiO2+2OH-=SiO32-+H2O��
��4�����ڿ�ʯ����FeCO3���������ӱ�����������ͬʱ���ᱻ��ԭ��һ����������Ӧ�����ӷ���ʽΪ��3FeCO3+10H++NO3-=3Fe3++3CO2��+NO��+5H2O��
�ʴ�Ϊ��3FeCO3+10H++NO3-=3Fe3++3CO2��+NO��+5H2O��
��5������NO���ж����壬���������ŷţ�������������Ŀ����NOѭ��ʹ���ܼ��ٻ�����Ⱦ����NO��H2O��O2��Ӧ���ֵõ��������ԭ�������ʣ��������Ϸ�����֪���ÿ�ʯ�к���̼�������Ͷ������裬����̼��������������2.36��103 kg-1.2��103 kg=1.16��103kg�����ʵ�����10000mol�������3FeCO3+10H++NO3-=3Fe3++3CO2��+NO��+5H2O�еķ���ʽ��֪��������������ʵ����ǣ�
| 105 |
| 3 |
�ʴ�Ϊ��NOѭ��ʹ���ܼ��ٻ�����Ⱦ��NO��H2O��O2��Ӧ���ֵõ��������ԭ�������ʣ�3��104��
�����������ۺϿ���ֹ���Ʊ���Ԫ�������ɵ�Ӧ�á����ʵļ��顢������ԭ��Ӧ����ʽ����д��β��������ԭ�ϵ�ʹ���Լ��йؼ��㣬�Ǹ߿��еij������ͣ��ѶȽϴ������ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ��������������ѧ�����������������ѧ��������û���֪ʶ���ʵ�������������

��ϰ��ϵ�д�
 ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ