��Ŀ����
�������ӷ���ʽ����д����۾���������
| ѡ�� | ���ӷ���ʽ | ���� |
| A | AgCl(s) + I-( aq)  AgI(s) + Cl-(aq) AgI(s) + Cl-(aq) | �ܽ�ȣ�AgI > AgCl |
| B | Fe2��+ H2O2 +2H��= Fe3��+2H2O | �����ԣ�H2O2 > Fe3�� |
| C | CO32- + CO2 + H2O = 2HCO3- | �ȶ��ԣ�HCO3- > CO32- |
| D | NH3 + H3O+=NH4++ H2O | ������������NH3> H2O |
D
Aѡ�����dz������ת����AgCl��ת��ΪAgI��˵���ܽ��AgI<AgCl��A������������������ǿ��Fe3+�������������ӷ���ʽ��ɲ��غ㣬B��������ת��Ϊ��ʽ�Σ��ȶ������δ�������ʽ��C��������NH3������ӵ������Ƚ�ǿ��NH3+H3O+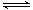 NH4++H2O��NH3�ܰ�ˮ�е���������ȥ������
NH4++H2O��NH3�ܰ�ˮ�е���������ȥ������
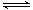 NH4++H2O��NH3�ܰ�ˮ�е���������ȥ������
NH4++H2O��NH3�ܰ�ˮ�е���������ȥ������
��ϰ��ϵ�д�
�����Ŀ
 S2-+ H3O+
S2-+ H3O+ H2�� + Cl2��
H2�� + Cl2��
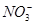 ===3Fe2���� 2NO�� �� 4H2O
===3Fe2���� 2NO�� �� 4H2O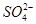 === 3BaSO4����2Al(OH)3��
=== 3BaSO4����2Al(OH)3�� Mn2����Cl2����2H2O
Mn2����Cl2����2H2O