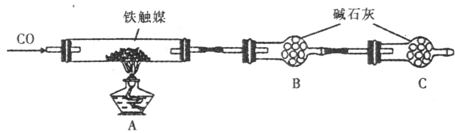
��Ŀ����
��16�֣�ijͬѧ���������˽�����и��������Ե��������Σ������κ�̼���Ρ���ͬѧ����ʵ����֤��һ��ʵ��������в����εĺ������������ϵ�֪���ᣨH2C2O4����һ�ֶ�Ԫ�л����ᣬ���н�ǿ�Ļ�ԭ�ԣ�����ƣ�CaC2O4��������ˮ������ϡ���ᡣ��ش��������⣺
��1����ѧ����������ĥ�ɷ�ĩ����ˮ���ݡ����˵õ�������ҺA����ĥ����ʹ�õ�ʵ������������Ϊ ��
��2�����ʵ�鷽����֤�����к��в����κ�̼���Σ��������Ԥ������ͽ��ۡ�
��3����ȷ�ⶨ�����в����εĺ���
�ٲ�����ȡm g������Ʒ�������в�����ȫ��ת��ΪCaC2O4����������������ձ��У��ù�����ϡ������ȫ�ܽ����Һת�� �в���ˮ���Ƴ�250mL��Һ��ÿ����ȡ25��00mL����Һ����ƿ�У���0��0100mol��L��1 KMnO4����Һ�ζ���ƽ�����ı���ҺV mL��KMnO4�ζ�ʵ��ʱ���������ӷ���ʽΪ ��
�ڼ��㣺�����в����Σ���C2O42�����㣩����������Ϊ������ֻ��ʽ������ ��
��������: ���в�����ʹ�ⶨ���ƫ�ߵ��� ��
A��������Һʱδϴ���ձ��Ͳ������ͼ�ˮ����
B����ƿδ����ͼ������Һ���еζ�
C��δ�ñ�Һ��ϴ�ͼ����Һ��ʼ�ζ�
D���ζ�ǰ���촦�����ݵζ���������ʧ
E���ζ����Ӷ���
��1����ѧ����������ĥ�ɷ�ĩ����ˮ���ݡ����˵õ�������ҺA����ĥ����ʹ�õ�ʵ������������Ϊ ��
��2�����ʵ�鷽����֤�����к��в����κ�̼���Σ��������Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��������ҺA�������ԣ��μ�����CaCl2��Һ�� | �ٳ��ְ�ɫ������˵�������� �� |
| ����2��ȡ����1�ij������Թ��У�����������ϡ���ᣬ������ȫ�ܽ⣬������������ɫ��ζ������ͨ�����ʯ��ˮ�С� | �� �� |
| ����3������2�õ�����Һ�еμӼ��θ��������Һ�� | �� �� |
�ٲ�����ȡm g������Ʒ�������в�����ȫ��ת��ΪCaC2O4����������������ձ��У��ù�����ϡ������ȫ�ܽ����Һת�� �в���ˮ���Ƴ�250mL��Һ��ÿ����ȡ25��00mL����Һ����ƿ�У���0��0100mol��L��1 KMnO4����Һ�ζ���ƽ�����ı���ҺV mL��KMnO4�ζ�ʵ��ʱ���������ӷ���ʽΪ ��
�ڼ��㣺�����в����Σ���C2O42�����㣩����������Ϊ������ֻ��ʽ������ ��
��������: ���в�����ʹ�ⶨ���ƫ�ߵ��� ��
A��������Һʱδϴ���ձ��Ͳ������ͼ�ˮ����
B����ƿδ����ͼ������Һ���еζ�
C��δ�ñ�Һ��ϴ�ͼ����Һ��ʼ�ζ�
D���ζ�ǰ���촦�����ݵζ���������ʧ
E���ζ����Ӷ���
��1���в���2�֣���
��2�������ٺ���C2O42����CO32���е�һ�ֻ����ֽ��У�2�֣���
�ڳ���ʯ��ˮ����ǣ�˵�в����к���CO32����2�֣���
�۸��������ɫ��ȥ��˵�������к���C2O42����2�֣���
��3����250ml ����ƿ��2�֣���2MnO4����16H����5C2O42��=2Mn2����10CO2��+8H2O��2�֣�
��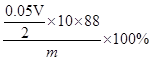 ��10-3��2�֣������������𰸣� ��CD��2�֣�
��10-3��2�֣������������𰸣� ��CD��2�֣�
��2�������ٺ���C2O42����CO32���е�һ�ֻ����ֽ��У�2�֣���
�ڳ���ʯ��ˮ����ǣ�˵�в����к���CO32����2�֣���
�۸��������ɫ��ȥ��˵�������к���C2O42����2�֣���
��3����250ml ����ƿ��2�֣���2MnO4����16H����5C2O42��=2Mn2����10CO2��+8H2O��2�֣�
��
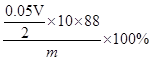 ��10-3��2�֣������������𰸣� ��CD��2�֣�
��10-3��2�֣������������𰸣� ��CD��2�֣������������1��������ĥ�õ�������Ϊ�в�����ĥ����ʹ�õ�ʵ������������Ϊ�в�����2������ƺ�̼��ƾ�Ϊ������ˮ�İ�ɫ���壬����1�г��ְ�ɫ������˵�����������ٺ���C2O42����CO32���е�һ�ֻ����ֽ��У�����2��Ŀ������֤�����к���̼���Σ�ѡ��ϡ�����ܽ�����������ǣ���������1 mol?L-1 HCl��������������ͨ����������ʯ��ˮ�У������dz����ܽ⣬����ʯ��ˮ����ǣ�˵�������к���̼���Σ�����3����Ŀ������֤�����εĴ��ڣ����ò����ξ��л�ԭ�ԣ������ǣ�����2�õ�����Һ�еμӼ���0.01 mol?L-1 KMnO4����������Һ�Ϻ�ɫ��ȥ��˵�������к��в����Σ����Ǣ����ٺ���C2O42����CO32���е�һ�ֻ����ֽ��С��ڳ���ʯ��ˮ����ǣ�˵�в����к���CO32�����۸��������ɫ��ȥ��˵�������к���C2O42������3��������250mL��ҺӦѡ��250mL����ƿ��KMnO4����Һ��C2O42����Ӧ����+2�������ӡ�������̼��ˮ�����ӷ���ʽΪ2MnO4����16H����5C2O42��=2Mn2����10CO2��+8H2O���ڼ��㣺���ݷ�Ӧ����ʽ�ù�ϵʽ��5C2O42-����2KMnO4������������ݼ��㣬�����в����Σ���C2O42�����㣩����������Ϊ
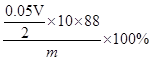 ��10-3����������: A��������Һʱδϴ���ձ��Ͳ������ͼ�ˮ���ݣ����������Һ�����ƫС���ⶨ���ƫ�ͣ�����B����ƿδ����ͼ������Һ���еζ����Բⶨ�����Ӱ�죬����C��δ�ñ�Һ��ϴ�ͼ����Һ��ʼ�ζ������������Һ�����ƫ�ⶨ���ƫ�ߣ���ȷ��D���ζ�ǰ���촦�����ݵζ���������ʧ�����������Һ�����ƫ�ⶨ���ƫ�ߣ���ȷ��E���ζ����Ӷ��������������Һ�����ƫС���ⶨ���ƫ�ͣ�����ѡCD��
��10-3����������: A��������Һʱδϴ���ձ��Ͳ������ͼ�ˮ���ݣ����������Һ�����ƫС���ⶨ���ƫ�ͣ�����B����ƿδ����ͼ������Һ���еζ����Բⶨ�����Ӱ�죬����C��δ�ñ�Һ��ϴ�ͼ����Һ��ʼ�ζ������������Һ�����ƫ�ⶨ���ƫ�ߣ���ȷ��D���ζ�ǰ���촦�����ݵζ���������ʧ�����������Һ�����ƫ�ⶨ���ƫ�ߣ���ȷ��E���ζ����Ӷ��������������Һ�����ƫС���ⶨ���ƫ�ͣ�����ѡCD��
��ϰ��ϵ�д�
�����Ŀ