��Ŀ����
����98%��ŨH2SO4��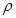 =1��84g/cm3�����Ƴ�Ũ��Ϊ0��5mol/L��ϡ����500mL��
=1��84g/cm3�����Ƴ�Ũ��Ϊ0��5mol/L��ϡ����500mL��
��1���뽫���в�������ȷ��������ں����ϣ�
A������Ͳ��ȡŨH2SO4
B�������ߵ�ҡ��
C���ý�ͷ�ιܼ�ˮ���̶�
D��ϴ���ձ��ڱںͲ�����������ϴҺת������ƿ
E��ϡ��ŨH2SO4
F������Һת������ƿ
�������ȷ˳��Ϊ_____________
��2����Ҫ�ش��������⣺
������ŨH2SO4�����Ϊ________mL��
�����ʵ������15mL, 20mL, 25mL����Ͳ��ѡ��_______mL����Ͳ��á���ȡʱ������Ͳ���ɾ�����ˮϴ����ֱ����ȡ��ʹʵ�����ս��______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�۽�ŨH2SO4���ձ��ڱ�����ע��ʢˮ���ձ��У����Ͻ����Ŀ����_______���������������Һ�彦������ʹ���ս��_______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ת������ƿǰ�ձ���Һ��Ӧ_______�������ʹŨ��_______��ϴ���ձ�2��3�Σ�ϴҺҲҪת������ƿ�������ʹ���ս��_______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�ݶ���ʱ����ʹ��Һ��Һ����̶������У������ӻ�ʹ���_______�����ӻ�ʹ���_______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��1��AEFDCB����2����13��6 ��15 ƫ�� �۷�ֹ���У�ʹϡ��Ũ���������������ʱɢ����ȥ ƫ�� �� ������ȴ ƫ�� ƫ�� ��ƫ�� ƫ�ͣ�ÿһ����1�֣�
��������
�����������1�����⿼��һ�����ʵ���Ũ����Һ���ƵIJ������裻���������м��㡢��ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������������ȷ˳��ΪAEFDCB����2���ٸ��� ����ŨH2SO4�����ʵ���Ũ��Ϊ18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬��Ũ��������ΪxmL������xmL��18.4mol/L=500mL��0.5mol/L����ã�x=13.6mL������ȡ13.6mLŨ���ᣬ��Ҫʹ��15mL����Ͳ����ȡʱ������Ͳ���ɾ���ˮϴ����ֱ����ȡ��Ũ���ᱻ����ˮϡ�ͣ�����Ũ�����Ũ��ƫ�ͣ���ʹʵ�����ս��ƫ�ͣ���Ũ����ϡ�ͷ��ȣ���ŨH2SO4���ձ��ڱ�����ע��ʢˮ���ձ��У����Ͻ����Ŀ���Ƿ�ֹ���У�ʹϡ��Ũ���������������ʱɢ����ȥ���������������Һ�彦����������ʧ����ʹ���ս��ƫ�ͣ���Ũ����ϡ�ͷ��ȣ���Һ���¶����ߣ�ת��ǰ������ȴϡ�͵���Һ��������ȴ�����Ƶ���Һ�����ƫ�ͣ����յ���Ũ��ƫ�ߣ�ϴ���ձ��Ͳ�����2��3�Σ�ϴҺҲҪת������ƿ�������ʹ���Ƶ���Һ�����ʵ����ʵ�����С��Ũ��ƫ�ͣ��ݶ���ʱ���ӣ�������Һ���ƫС��������Һ��Ũ��ƫ�ߣ������ӣ�������Һ�����ƫ�����Ƶ���ҺŨ��ƫ�͡�
����ŨH2SO4�����ʵ���Ũ��Ϊ18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬��Ũ��������ΪxmL������xmL��18.4mol/L=500mL��0.5mol/L����ã�x=13.6mL������ȡ13.6mLŨ���ᣬ��Ҫʹ��15mL����Ͳ����ȡʱ������Ͳ���ɾ���ˮϴ����ֱ����ȡ��Ũ���ᱻ����ˮϡ�ͣ�����Ũ�����Ũ��ƫ�ͣ���ʹʵ�����ս��ƫ�ͣ���Ũ����ϡ�ͷ��ȣ���ŨH2SO4���ձ��ڱ�����ע��ʢˮ���ձ��У����Ͻ����Ŀ���Ƿ�ֹ���У�ʹϡ��Ũ���������������ʱɢ����ȥ���������������Һ�彦����������ʧ����ʹ���ս��ƫ�ͣ���Ũ����ϡ�ͷ��ȣ���Һ���¶����ߣ�ת��ǰ������ȴϡ�͵���Һ��������ȴ�����Ƶ���Һ�����ƫ�ͣ����յ���Ũ��ƫ�ߣ�ϴ���ձ��Ͳ�����2��3�Σ�ϴҺҲҪת������ƿ�������ʹ���Ƶ���Һ�����ʵ����ʵ�����С��Ũ��ƫ�ͣ��ݶ���ʱ���ӣ�������Һ���ƫС��������Һ��Ũ��ƫ�ߣ������ӣ�������Һ�����ƫ�����Ƶ���ҺŨ��ƫ�͡�
���㣺����һ�����ʵ���Ũ����Һ�����ơ�