��Ŀ����
A��J����ѧ��ѧ�г����ļ������ʣ�����֮���ת����ϵ��ͼ��ʾ����֪������AΪ���嵥�ʣ�BΪ����ɫ��ĩ�� C��F��IΪ��̬���ʣ�E�ڳ�����ΪҺ�壬��E����C��F�ϳɣ�J������ɱ�����������ش��������⣺
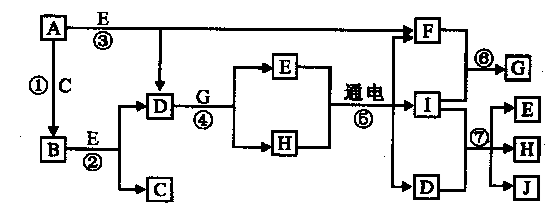
��1��B�������ӵĵ���ʽΪ ����E���Ԫ����ͬ�Ļ�����ĽṹʽΪ ��
��2����֪D��G��Ӧ����ImolE�ų�������ΪaKJ����д����ʾD��H2SO4�к��ȵ��Ȼ�ѧ����ʽ ��
��3����FeCl2��Һ�м����������B��д����Ӧ���ӷ���ʽ ��
��4����������PtΪ�缫���μ���������̪��H������Һ2L������ ���������������������Һ����ɫ��Ϊ��ɫ������F��C��ɵ�أ�����K2CO3������ʣ����磬��صĸ�����ӦʽΪ ��������ӦʽΪ ���������У�������Һ����仯������Һ��PH=13ʱ�������������������ڱ����Ϊ ��
��1��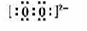 ��2�֣��� H��O��O��H��2�֣�
��2�֣��� H��O��O��H��2�֣�
��2��NaOH��aq��+1/2H2SO4��aq��=1/2Na2SO4��aq��+H2O��l����H=��aKJ/mol��2�֣�
��3��4Fe2++4Na2O2+6H2O=4Fe��OH��3+O2��+8Na+��2�֣�
��4������1�֣���2H2��4e-+2CO32-=2H2O+2CO2����H2��2e-+CO32-=H2O+CO2������2�֣�O2+4e-+2CO2=2CO32-����2�֣�2.24L��2�֣�
��������
���������BΪ����ɫ��ĩ���ƶ�B��Na2O2��E�ڳ�����ΪҺ�壬E��H2O����֪A��Na��C��O2��F��H2��D��NaOH��J������ɱ�����������ƶ�J��NaClO����֪H��NaCl��G��HCl��I��Cl2��
B����������O22-������ʽΪ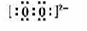 ��E���Ԫ����ͬ����H2O2�ṹʽΪH��O��O��H��
��E���Ԫ����ͬ����H2O2�ṹʽΪH��O��O��H��
��2��NaOH��HCl��Ӧ����1molˮ�ų�����ΪaKJ/mol��NaOH�����ᷴӦ����1molˮ�ų���������ͬ�ģ�����ȷ���ΪNaOH��aq��+1/2H2SO4��aq��=1/2Na2SO4��aq��+H2O��l����H=��aKJ/mol��
��FeCl2��Һ�м������Na2O2��Na2O2��ˮ��Ӧ����NaOH��O2��Fe2+������ΪFe(OH)3�����ӷ���ʽΪ4Fe2++4Na2O2+6H2O=4Fe��OH��3+O2��+8Na+
���NaCl��Һ������H+�õ��ӷų�������ʹˮ�ĵ���ƽ�������ƶ���C(OH-)>C(H+)����Һ�Լ��ԣ�H2��O2������K2CO3����ȼ�ϵ�أ�H2����������������Ӧ��O2������������ԭ��Ӧ��2H2��4e-+2CO32-=2H2O+2CO2 O2+4e-+2CO2=2CO32-������Һ��PH=13ʱ��C(OH-)=0.1mol/L��ÿ����1molOH-ת��1mole-������1molH+������0.5molH2����ˣ�n(H2)=0.5n(OH-)=0.1��2��0.5=0.1mol�����Ϊ2.24L��
���㣺����Ԫ�ػ���������ʡ���ѧ�������ʽ����д���绯ѧ�����ʵ����ļ���






