��Ŀ����
�±���Ԫ�����ڱ���һ����
��1������Ԫ�آ���⻯��Ļ�ѧʽΪ���������������������⻯��Ļ�ԭ�Ա�Ԫ�آ���⻯��Ļ�ԭ�������������ǿ������������
��2��ijԪ��ԭ�ӵĺ���p��������s��������1�����Ԫ�ص�Ԫ�ط��������������䵥�ʵĵ���ʽΪ��������������������
��3���׳�Ϊ��������һ�����ͨ�����еĹ�ͬ��Ԫ��������������������������
��4����֪ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ���Ԫ�آ���Ԫ�آ���������������Ƶ����ʡ�д��Ԫ�آ۵�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
�������������������������������������������������Ԫ�آߵ��������ƵIJ�ͬ���Ԫ������������������Ԫ�ط��ţ���
| | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
| 1 | �� | | | | | | |
| 2 | �� | �� | �� | �� | �� | | |
| 3 | | �� | �� | | | �� | �� |
��2��ijԪ��ԭ�ӵĺ���p��������s��������1�����Ԫ�ص�Ԫ�ط��������������䵥�ʵĵ���ʽΪ��������������������
��3���׳�Ϊ��������һ�����ͨ�����еĹ�ͬ��Ԫ��������������������������
��4����֪ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ���Ԫ�آ���Ԫ�آ���������������Ƶ����ʡ�д��Ԫ�آ۵�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
�������������������������������������������������Ԫ�آߵ��������ƵIJ�ͬ���Ԫ������������������Ԫ�ط��ţ���
��1��HCl����2��N����
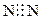 ���3��H��O��S
���3��H��O��S��4��Be(OH)2+2NaOH
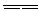 Na2BeO2+2H2O
Na2BeO2+2H2O��1�����ݢ����������ں�����������ΪCl����ԭ��HCl<H2S��
��2����ֻ��1���ܲ㣬����p�ܼ�������2���ܲ㣬��������s�ܼ���������Ų�Ϊ1s22s22p3ΪNԪ�أ�����3���ܲ㣬��������s�ܼ������������⣻
��3�������εĽᾧˮ�����׳Ʒ�������CuSO4��5H2O��������������һ�����ͨ�����й�ͬ��Ԫ����H��O��S��
��4�����⿼��Խ��߹���ijЩ����Ԫ�������·�������Ԫ�ص���Щ�������ơ�����Ϊ�Խ��ߣ��������ƣ���ΪBe����ΪAl��Be����������Ҳ�������ԣ�����ǿ���ǿ�Ӧ��Be(OH)2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��Be(OH)2+2NaOH
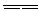 Na2BeO2+2H2O������ڻ�Ϊ�Խ��ߣ�����Li��
Na2BeO2+2H2O������ڻ�Ϊ�Խ��ߣ�����Li��
��ϰ��ϵ�д�
�����Ŀ
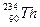 ��ԭ����˵�� ��
��ԭ����˵�� ��