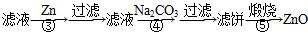��Ŀ����
�������(K2FeO4)�����Ͷ��ˮ�����������������������ȶ����������������£�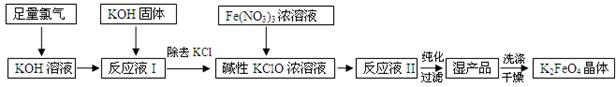
��ش���������
��1���ȼҵ��Cl2�Ļ�ѧ��Ӧ����ʽ ��
��2�����ɡ���ӦҺ�����ӷ���ʽ�� ����3��ʹ�ü���KClO��ԭ���� ��
��4���ӡ���ӦҺII���з����K2FeO4�� ������Ʒ��KCl�� (�ѧʽ)���û������� ���������ᴿ������ĸ��ţ���
| A������ | B����Һ | C������ | D���ؽᾧ |
��1��2NaCl + 2H2O 2NaOH + H2��+ Cl2��
2NaOH + H2��+ Cl2��
��2��3ClO- + 2Fe3+ + 10OH- = 2FeO42- + 3Cl- + 5H2O��
��3��K2FeO4�ڼ����������ȶ���KClO������ǿ��K2FeO4
��4�� KNO3 �� D
��5�� 3.00 �� 104
���������������1���ȼҵ���ǵ�ⱥ��ʳ��ˮ�õ��ռ����������2���������̷�����ӦҺII�����ô���������������ӵõ�FeO42-�����������ԭ��Ӧ���ӷ���ʽ����д����ɵã�3ClO- + 2Fe3+ + 10OH- = 2FeO42- + 3Cl- + 5H2O����3����Ŀ��Ϣ��K2FeO4�ڼ����������ȶ�����Ӧ���ڼ��������½��У���4����Ӧ�м����������������Ի�����KNO3������ص��ܽ�����¶ȱ仯�ϴ�������ȴ�ᾧ�ķ������룻��5������������Ӧ�ʹ����������������Ӧ�Ĺ�ϵʽ���м��㡣
���㣺�����ɢϵ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д��ú���A1203��SiO2������FeO��xFe2O3�������Ʊ�A12(SO4)3��18H2O�������������£�
��һ�������£�MnO4 - ����Mn2+��Ӧ����MnO2��
��֪�������������������pH
| | Al��OH��3 | Fe��OH��2 | Fe��OH��3 |
| ��ʼ����ʱ | 3��4 | 6��3 | 2��7 |
| ��ȫ����ʱ | 5��2 | 9��7 | 3��2 |
��1��H2S04�ܽ�A1203�����ӷ���ʽ��
��2��������Һ�л�����Fe2���ķ����� (ע���Լ�������)��
��3�������ӡ����������¼������裬��������Һ�м������KMnO4��Һ��������Һ��pHΪ3��2�������ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ:�� ����MnSO4���Ϻ�ɫ��ʧ�����ˡ�
�ٲ�����Ŀ�ģ� ��������Һ��pHΪ3��2��Ŀ���� ��
�����ij����м���ŨHCl�����ȣ���˵�������д���MnO2�������� ��д���䷴Ӧ����ʽ�� ��
�ۢ��м���MnS04��Ŀ���� ��
��4���Ӷ��ѭ��ʹ�ú�ĸҺ�пɻ��յ���Ҫ������ �����ѧʽ��
��ҵ�����÷���м�����������������������ȣ�������ʽ������[Fe(OH)SO4]�Ĺ����������£�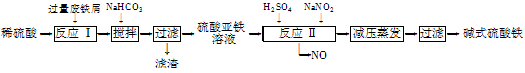
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
�ش��������⣺
��1�������������м��Ŀ���� ��
��2����������NaHCO3��Ŀ���ǵ���pH��ʹ��Һ�е�________��ѡ�Fe3��������Fe2������Al3�������������ù��������С����衱�������� ��
��3����Ӧ������ӷ���ʽΪ ����ʵ�������У���Ӧ��ͬʱͨ��O2�Լ���NaNO2��������O2��NaNO2�ڷ�Ӧ�о��� �������뷴Ӧ��O2��11.2 L����״���������൱�ڽ�ԼNaNO2�����ʵ���Ϊ ��
��4����ʽ����������ˮ�������Fe(OH)2�����ӣ��ɲ���ˮ������Fe2(OH)42+�ۺ����ӡ���ˮ�ⷴӦ�����ӷ���ʽΪ ��
��5����ҽҩ�ϳ����������������ᡢ����Ļ��Һ��Ӧ�Ʊ���ʽ�������������ҹ�����������Ʒ�в��ú���Fe2����NO3-��Ϊ�������ò�Ʒ���Ƿ���Fe2����Ӧʹ�õ��Լ�Ϊ ��
A����ˮ B��KSCN��Һ C��NaOH��Һ D������KMnO4��Һ
��6����11.9 g Mg��Al��Fe��ɵĺϽ�����������NaOH��Һ�У��Ͻ�����������2.7 g����ȡ�������ĺϽ����ڹ���ϡ�����У�������6.72 L����״���£�NO����Ӧ�����Һ�м�������NaOH��Һǡ��ʹMg2����Al3����Fe3����ȫת��Ϊ���������������Ϊ ��
A��22.1 g B��27.2 g C��30 g D����ȷ��
ϡ��Ԫ�������ڱ��Т� B���֡��ƺ���ϵԪ�ص��ܳƣ����Ƕ��Ǻܻ��õĽ��������ʼ�Ϊ���ƣ��������ϼ�Ϊ+3�������ƣ�Y��Ԫ���Ǽ���ͳ�������Ҫ���ϡ��ҹ��̲��ŷḻ���ƿ�ʯ�� Y2FeBe2Si2O10�����Դ˿�ʯΪԭ�����������ƣ�Y2O3������Ҫ�������£�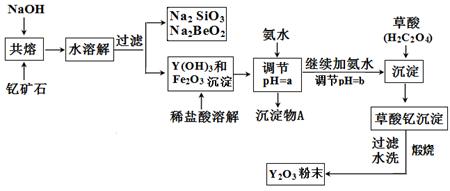
��֪��I���йؽ��������γ������������ʱ��pH���±���
| | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe3+ | 2.7 | 3.7 |
| Y3+ | 6.0 | 8.2 |
�������ڱ��У��롢��Ԫ�ش��ڵڶ����ں͵������ڵĶԽ���λ�ã���ѧ�������ơ�
��1���ƿ�ʯ��Y2 FeBe2Si2O10������������������ʽ�ɱ�ʾΪ ��
��2������Na2 SiO3��Na2BeO2�Ļ����Һ���Ƶ�Be(OH)2��������
�����ѡ�����������ѡ���е� �Լ�������ĸ���ţ���
a��NaOH��Һ b����ˮ c��CO2�� d��HNO3
��ͨ�� ��������ʵ�֣���ʵ��������ƣ���
��д��Na2BeO2���������ᷢ����Ӧ�����ӷ���ʽ�� ��
��3��ΪʹFe3+������ȫ�����ð�ˮ����pH =a����aӦ������
�ķ�Χ�ڣ������Ӱ�ˮ����pH =b������Ӧ�����ӷ���ʽΪ ����Һ��Fe3+��ȫ�������ж����� ��
����ʯ����Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽�����£�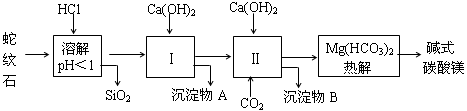
��1������ʯ��������ܽ����Һ�����Mg2+�⣬�����еĽ��������� ��
��2�����Т����ʱ��������Һ��pH=7~8���й��������������pH���±�����Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ�� �ܽ⣬���� ������
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
��3���ӳ��������A����ȡ��ɫ��������Ϊ���ϣ����������A�м��� ����������ʵĻ�ѧʽ����Ȼ�� ��������дʵ��������ƣ�������ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ������� ����д���ʵĻ�ѧʽ����
��4�������ʵ�飬ȷ����ƷaMgCO3��bMg(OH)2��cH2O��a��b��c��ֵ����д������ʵ�鲽��������Ҫ�ⶨ����Ŀ�������Լ���Ũ���ᡢ��ʯ�ҡ�����������Һ������ʯ��ˮ��������Ʒ�������ڸ��·ֽ⣬�� ���� ����MgO������
��5��������������װ�б�Ҫ���Լ�����ѡ���������ʵ�������������������һ��װ�� ��ѡ���������ţ����ظ�ʹ�ã��á�A��B������������ʾ��

��6��18.2g��Ʒ��ȫ�ֽ����6.6gCO2��8.0gMgO���ɴ˿�֪����Ʒ�Ļ�ѧʽ��a= ��b= ��c= ��
 MnO2+Zn+2H2SO4��
MnO2+Zn+2H2SO4��