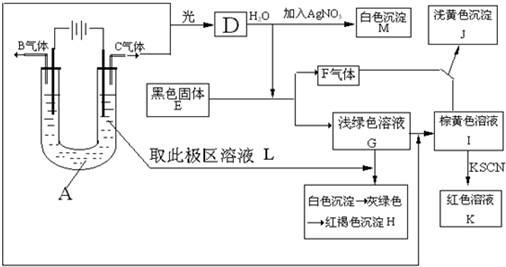
��Ŀ����
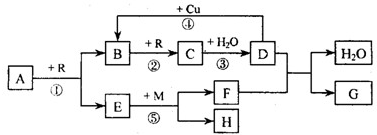
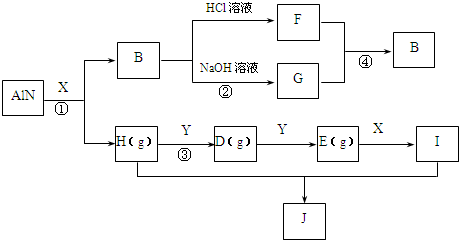
��������A-I����ת����ϵ��ͼ����֪��AlNΪ��ˮ��Ļ����X������Ϊ��ɫ��ζ��Һ�壬B�Dz�����ˮ�İ�ɫ��״������H������ʹʪ��ĺ�ɫʯ����ֽ������E�ڳ�����Ϊ����ɫ���壬��Ϊһ�ֳ����Ĵ�����Ⱦ������ַ�Ӧ��������������ȥ��
��������ת����ϵͼ�ش�
��1��д���������ʵĻ�ѧʽ X______��B______��J______��
��2����֪4molH��g����5molY��g������D��g����X��l�����ų�a kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��______�����þ������ʽ��
��3��д����Ӧ�ڵ����ӷ���ʽ______��

��������ת����ϵͼ�ش�
��1��д���������ʵĻ�ѧʽ X______��B______��J______��
��2����֪4molH��g����5molY��g������D��g����X��l�����ų�a kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��______�����þ������ʽ��
��3��д����Ӧ�ڵ����ӷ���ʽ______��
X������Ϊ��ɫ��ζ��Һ�壬ӦΪH2O��AlN��H2O������AlN+3H2O=Al��OH��3��+NH3����B�Dz�����ˮ�İ�ɫ��״������H������ʹʪ��ĺ�ɫʯ����ֽ��������BΪAl��OH��3��HΪNH3����FΪAlCl3��GΪNaAlO2��E�ڳ�����Ϊ����ɫ���壬��Ϊһ�ֳ����Ĵ�����Ⱦ�ӦΪNO2����DΪNO��YΪO2��IΪHNO3��JΪNH4NO3����
��1�������Ϸ�����֪XΪH2O��BΪAl��OH��3��JΪNH4NO3���ʴ�Ϊ��H2O��Al��OH��3��NH4NO3��
��2��HΪNH3��YΪO2�����߷�Ӧ����ʽΪ4NH3+5O2
4NO+6H2O���ų�a kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ
4NH3��g��+5O2��g��=4NO��g��+6H2O��l����H=akJ/mol��
�ʴ�Ϊ��4NH3��g��+5O2��g��=4NO��g��+6H2O��l����H=akJ/mol��
��3����Ӧ��ΪAl��OH��3��NaOH�ķ�Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��1�������Ϸ�����֪XΪH2O��BΪAl��OH��3��JΪNH4NO3���ʴ�Ϊ��H2O��Al��OH��3��NH4NO3��
��2��HΪNH3��YΪO2�����߷�Ӧ����ʽΪ4NH3+5O2
| ||
| ���¸�ѹ |
4NH3��g��+5O2��g��=4NO��g��+6H2O��l����H=akJ/mol��
�ʴ�Ϊ��4NH3��g��+5O2��g��=4NO��g��+6H2O��l����H=akJ/mol��
��3����Ӧ��ΪAl��OH��3��NaOH�ķ�Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��

��ϰ��ϵ�д�
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
�����Ŀ