��Ŀ����
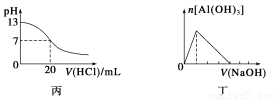
�ܱ������г���10 mol CO��20 mol H2���ڴ��������·�Ӧ���ɼ״���CO(g)��2H2(g) CH3OH(g)��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ������˵����ȷ���ǣ� ��
CH3OH(g)��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ������˵����ȷ���ǣ� ��
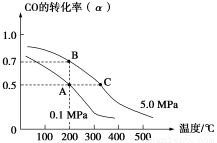
A����A��B�����ʾ��ijʱ�̴ﵽ��ƽ��״̬����ʱA��B����ʱ�����У�n(A)����n(B)����4��5
B����A��C���㶼��ʾ�ﵽ��ƽ��״̬�����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��tA��tC
C����B��C�����ƽ�ⳣ���ֱ�ΪKB��KC����KB��KC
D���ڲ��ı䷴Ӧ������������£����¡���ѹ�����״��ӻ����ϵ�з�������������CO��ת����
D
���������� CO(g)��2H2(g)  CH3OH(g)
CH3OH(g)
��ʼ/mol 10 20 0
Aƽ��/mol 5 10 5
Bƽ��/mol 3 6 7
ѡ��A�У�n(A)����n(B)����20��16��5��4��ѡ��A����A���C����ȣ�C����¶ȸߡ�ѹǿ��Ӧ���ʿ죬����ƽ���ʱ��̣�tA��tB��ѡ��B����B��C�㣬�¶����ߣ�ƽ�����ƣ�K��С����KB��KC��ѡ��C�����¡���ѹ������������Ũ�ȶ�ʹƽ�����ƣ�CO��ת��������ѡ��D��ȷ��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�һ���¶��£����Ϊ2 L���ܱ�������X��Y��Z��������ij�ʼ���ʵ�����ƽ�����ʵ������±���
���� | X | Y | Z |
��ʼ���ʵ���(mol) | 0.2 | 0.2 | 0 |
ƽ�����ʵ���(mol) | 0.1 | 0.05 | 0.1 |
����˵����ȷ���ǣ� ��
A����Ӧ�ɱ�ʾΪ2X��3Y 2Z����ƽ�ⳣ��Ϊ8 000
2Z����ƽ�ⳣ��Ϊ8 000
B������ѹǿʹƽ��������Z�ķ����ƶ���ƽ�ⳣ������
C���������������ѹ����1 L����X�����������С��Ũ������
D���������¶�ʱ��Z��Ũ������֪�¶�����ʱ����Ӧ���������淴Ӧ���ʼ�С��ƽ��������Ӧ�����ƶ�
һ���¶��£���2 L�ܱ������з������з�Ӧ��4NO2(g)��O2(g) 2N2O5(g)����֪�÷�Ӧ��ƽ�ⳣ����K300 ����K350 ������n(NO2)(��λ��mol)��ʱ��仯���±���
2N2O5(g)����֪�÷�Ӧ��ƽ�ⳣ����K300 ����K350 ������n(NO2)(��λ��mol)��ʱ��仯���±���
ʱ��(s) | 0 | 500 | 1000 | 1500 |
t1�� | 20 | 13.96 | 10.08 | 10.08 |
t2�� | 20 | a | b | c |
����˵��һ����ȷ���ǣ� ��
A������ӦΪ���ȷ�Ӧ
B�����t2����t1������ôa��b��c����a��10��0.5b
C�����t2����t1������ôt2���ﵽƽ���ʱ�����1 000 s��1 500 s֮��
D�����t2����t1������ôb��10.08