��Ŀ����
��ӦN2+3H2![]() 2NH3 �SH= ��92��4 kJ/mol ��һ���¶Ⱥ����������ܱ������н��в��ﵽƽ�⣬������c (N2 )= 2mol/L��c (H2)=5mol/L����Ӧ�ﵽƽ��ʱ��һ���N2�����˷�Ӧ����
2NH3 �SH= ��92��4 kJ/mol ��һ���¶Ⱥ����������ܱ������н��в��ﵽƽ�⣬������c (N2 )= 2mol/L��c (H2)=5mol/L����Ӧ�ﵽƽ��ʱ��һ���N2�����˷�Ӧ����
��1����ﵽƽ��ʱ�� H2��NH3��Ũ�ȷֱ�Ϊc (H2 )= mol/L��c (NH3)= mol/L
��2����ƽ��ʱH2��ת����Ϊ ����ƽ��ʱNH3�����ʵ�����ƽ��������ʵ����ı�ֵΪ
��3������¶��µ�ƽ�ⳣ��Ϊ ���ﵽƽ�������¶ȣ���ƽ�� �������ƣ����ƻ��ƶ�����Kֵ ��H2��ת���� ����������������С�䣩
��1��c (H2 )= 2 mol/L��c (NH3)= 2 mol/L
��2�� 60% 0.4��2/5
��3�� 0.5 ����
��С ��С
����:
��

��ϰ��ϵ�д�
 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
�����Ŀ
 2NH3����10s��N2��Ũ����5mol/L����4mol/L����˵����ȷ���ǣ�������
2NH3����10s��N2��Ũ����5mol/L����4mol/L����˵����ȷ���ǣ������� 2NH3 ���в��Ǹ÷�Ӧ�ﵽƽ��״̬��־���ǣ�������
2NH3 ���в��Ǹ÷�Ӧ�ﵽƽ��״̬��־���ǣ������� 2NH3�������й��ƶ���ȷ���ǣ�������
2NH3�������й��ƶ���ȷ���ǣ�������
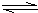 2NH3�ġ�H
2NH3�ġ�H 2NH3������ѡ�����Ϊ��Ӧ�ﵽƽ��״̬��־���ǣ�������
2NH3������ѡ�����Ϊ��Ӧ�ﵽƽ��״̬��־���ǣ�������