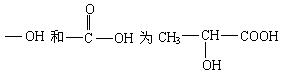��Ŀ����
12gij�л���X��ȫȼ�պ�����17.6 g CO2��7.2g H2O
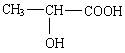
��1�����X���д̼�����ζ�����壬���Ҿ��л�ԭ�ԣ������Ľṹ��ʽΪ________________��
��2�����X���д̼�����ζ����ɫҺ�壬�������ơ�̼���ƾ��ܷ�Ӧ���ų����壬�����Ľṹ��ʽΪ________��
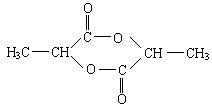
��3�����X���ӷ����й�����ζ����ɫҺ�壬����ˮ�⣬�����Ľṹ��ʽΪ________��
��4�����X�ķ�����Ϊ90�����ܸ�������������Ӧ���ܸ����ᷢ��������Ӧ������������X֮��Ҳ�ܻ����������Ӧ���ɻ�״����
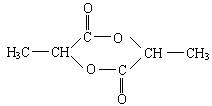
��X�Ľṹ��ʽΪ________��
��5�����X�ķ�������6��̼ԭ�ӣ��Ⱦ��ж�Ԫ�������ʣ��־��л�ԭ�ԣ������Ľṹ��ʽΪ________________��
�𰸣�
������
������
��1�� ��2��CH3COOH ��3�� ��4�� ��5��CH2OH(CHOH)4CHO �����������ʽ n(C)��n(H)��n(O)= �� ���ʽΪCH2O ��1���� ��2������Na2CO3��Ӧ֤���� ��3����ˮ����ζ������������Ϊ ��4��������ͬʱ���� ��5��Ϊ������CH2OH(CHOH)4CHO
|

��ϰ��ϵ�д�
�����Ŀ