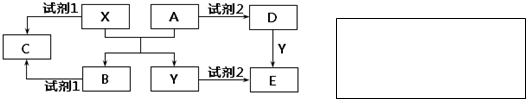
��Ŀ����
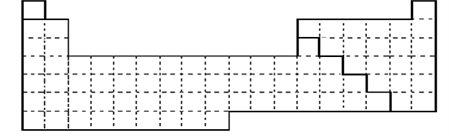
��ͼ��Ԫ�����ڱ��Ŀ��
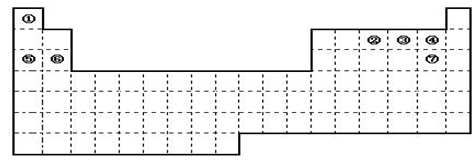
��1����������Ԫ�����ڱ��л�������Ԫ����ǽ���Ԫ�صķֽ��ߡ�
��2������Ԫ�����ڱ��ش��������⣺
A�����ڱ��е�Ԫ�آݺ�Ԫ�آ�����������ˮ�������ǿ��˳���� ���û�ѧʽ��ʾ����
B�����ڱ��е�Ԫ�آܺ�Ԫ�آߵ��⻯����ۡ��е�ߵ�˳���� ���û�ѧʽ��ʾ����
C���١���Ԫ�صĵ��ʣ��ڳ����»�ѧ�����ȶ���ͨ������������������ ����д�ṹʽ����
D��������Ԫ�����ڱ���ȫ���ǽ���Ԫ�ص������� ��ȫ���Ƿǽ���Ԫ�ص������� (��д��ĸa��b��c��d)��
a����A�� b����A�� c����A �� d����A��
��3����֪��Ԫ��λ�ڵ������ڣ�����ԭ�Ӱ뾶Ϊͬ���ڽ���Ԫ����ԭ�Ӱ뾶��С�ģ���д������������NaOH��Һ��Ӧ�����ӷ���ʽ ��
��4����д�����â٢ڢ�����Ԫ���γ����ӻ�����ĵ���ʽ_________���������ģ�ͱ�ʾ�ٺ͢��γɵĻ�����ķ��ӽṹ��Ӧ���� ��
��2������Ԫ�����ڱ��ش��������⣺
A�����ڱ��е�Ԫ�آݺ�Ԫ�آ�����������ˮ�������ǿ��˳���� ���û�ѧʽ��ʾ����
B�����ڱ��е�Ԫ�آܺ�Ԫ�آߵ��⻯����ۡ��е�ߵ�˳���� ���û�ѧʽ��ʾ����
C���١���Ԫ�صĵ��ʣ��ڳ����»�ѧ�����ȶ���ͨ������������������ ����д�ṹʽ����
D��������Ԫ�����ڱ���ȫ���ǽ���Ԫ�ص������� ��ȫ���Ƿǽ���Ԫ�ص������� (��д��ĸa��b��c��d)��
a����A�� b����A�� c����A �� d����A��
��3����֪��Ԫ��λ�ڵ������ڣ�����ԭ�Ӱ뾶Ϊͬ���ڽ���Ԫ����ԭ�Ӱ뾶��С�ģ���д������������NaOH��Һ��Ӧ�����ӷ���ʽ ��
��4����д�����â٢ڢ�����Ԫ���γ����ӻ�����ĵ���ʽ_________���������ģ�ͱ�ʾ�ٺ͢��γɵĻ�����ķ��ӽṹ��Ӧ���� ��
A��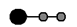 B��
B��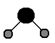 C��
C�� D��
D��
 B��
B�� C��
C�� D��
D��
�� ��120����1g�ٵĵ����������۵ĵ�������ȫȼ�գ��ų�������Ϊa kJ����д���������¢ٵĵ���ȼ�յ��Ȼ�ѧ����ʽ ���١�������Ԫ�صĵ����ѱ�Ӧ��������ɴ���ȼ�ϵ���У�����ͼ��ʾ�������缫���ɶ����̼���ɣ�ͨ������ֵ����ɿ�϶�ݳ����ڵ缫����ŵ硣 ��ش�b�ǵ�ص� ����a�缫�ϵĵ缫��Ӧʽ��_____________��
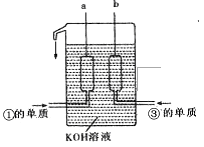
��1����ͼ��
��2��A��NaOH��Mg(OH)2��B��HF��HCl��C�� ��D��b��d
��D��b��d
��3��Al2O3 + 2OH��= 2AlO2��+ H2O
��4��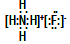 ��B
��B
��5��H2(g) + 1/2 O2(g) = H2O(l) ��H =��2akJ/mol��������H2 - 2e��+ 2OH��= 2H2O

��2��A��NaOH��Mg(OH)2��B��HF��HCl��C��
 ��D��b��d
��D��b��d ��3��Al2O3 + 2OH��= 2AlO2��+ H2O
��4��
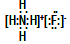 ��B
��B ��5��H2(g) + 1/2 O2(g) = H2O(l) ��H =��2akJ/mol��������H2 - 2e��+ 2OH��= 2H2O

��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д�
�����Ŀ