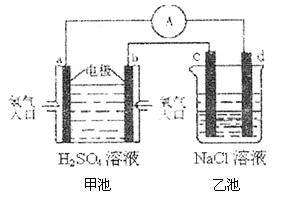
��Ŀ����
��һ�ݻ�Ϊ2.0L���ݻ�������ܱ������м�������̼�ۺ�0.20molH2O����800�������£���20 s��ﵽ���»�ѧƽ��(��һƽ��)��C(s)+H2O(g)![]() CO(g)+H2(g)��DH��0
CO(g)+H2(g)��DH��0
��֪ƽ��ʱ��COΪ0.12mol������գ�
(1)����v(H2O)��ʾ�÷�Ӧǰ20s��ƽ�����ʣ���v(H2O)=________��
(2)��������ƽ�������м�������Na2O���壬���ڴ��¶����ٴδﵽ�µ�ƽ��(�ڶ�ƽ��)����ƽ��ʱH2�����ʵ���________(�������С�����䡱)��������________��
(3)����������һƽ��Ļ�������ٳ���amolH2(a��0.12)����ͬ�����´ﵽ�µ�ƽ��(����ƽ��)����ʱCO�����ʵ���n�ķ�Χ��________��
������
| (1)0.0030mol��L-1��s-1
(2)��С,Na2O��H2O��Ӧ��ʹˮ����Ũ�Ƚ��ͣ�ƽ�������ƶ� (3)(0.12-a)��n��0.12 ������(1)20s����CO��ʾ�÷�Ӧ��ƽ������v(CO)=(0.12mol��2L)/20 s=0.003mol��L-1��s-1�� ���ݣ����ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ���H2O��ʾ�÷�Ӧǰ20s�ڵ�ƽ������v(H2O)=0.003mol��L-1��s-1�� (2)����Na2O��H2O��Ӧ��ʹˮ����Ũ�Ƚ��ͣ�ƽ�������ƶ�����˵ڶ���ƽ��ʱ��H2��Ũ�ȼ�С�� (3)��һ��ƽ��ʱCO��H2���ʵ�����Ϊ0.12mol������amolH2��ƽ�������ƶ���CO�����ʵ�����С���ﵽƽ��(����ƽ��)ʱ��CO�����ʵ���С��0.12mol��������������ԭ��������amolH2��ѧƽ����������ָı�ķ����ƶ�(���ܵ������ָı�)����˱仯��H2�����ʵ���С��amol������ƽ��ʱCO�����ʵ�������(0.12-a)mol��
|

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�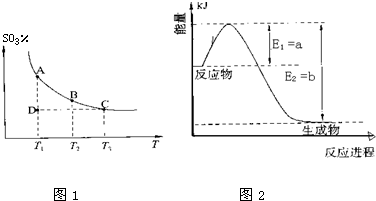

 2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��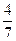 ������������´ﵽƽ��ʱN2ת����Ϊ ��
������������´ﵽƽ��ʱN2ת����Ϊ ��