��Ŀ����
���б���һԪ���Ļ����ͱ���һԪ����Ļ������һ�������·�Ӧ���ɶ������Ļ���������Է��������ֱ�ΪM1��M2��M3������������14������������Է�������ΪM2������������Ԫ����ռ����������Ϊ31.4%��������������ͬ���칹�塣
������������⣺
��1����Է�������ΪM2�����ķ���ʽΪ______________________________��
��2������һԪ����ķ���ʽ�ֱ�Ϊ______________________��______________________��
��3���μӷ�Ӧ�Ĵ��Ľṹ��ʽΪ_______________��_______________��_______________��_______________��������������
��4������Է�������ΪM1������Ϊͬ���칹�壬�ܷ���������Ӧ����������Ʒ�Ӧ�����������л����ͬ���칹����_______________�֡�
��1��C5H10O2
��2��C2H4O2 C3H6O2
��3��CH3CH2CH2OH��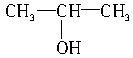 ��CH3CH2OH
��CH3CH2OH
��4��5
����:
���ݱ�������ͨʽCnH2nO2����Է�������ΪM2�����е�������������Ϊ31.4%�����У�
![]() ��100%=31.4% n=5,�������ʵķ���ʽΪC5H10O2�����ڸ�����ֻ����������ͬ���칹�壬M1��M2��M3��������14����ֻ����CH3CH2COOC2H5��CH3COOCH2CH2CH3��CH3COOCH��CH3��2����Ӧ������ֱ���CH3CH2COOH��CH3COOH����Ӧ�Ĵ��ֱ���C2H5OH��CH3CH2CH2OH��
��100%=31.4% n=5,�������ʵķ���ʽΪC5H10O2�����ڸ�����ֻ����������ͬ���칹�壬M1��M2��M3��������14����ֻ����CH3CH2COOC2H5��CH3COOCH2CH2CH3��CH3COOCH��CH3��2����Ӧ������ֱ���CH3CH2COOH��CH3COOH����Ӧ�Ĵ��ֱ���C2H5OH��CH3CH2CH2OH��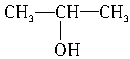 ����Է�������ΪM1�����ʵķ���ʽΪC4H8O2��������������ĸ��л����ͬ���칹����
����Է�������ΪM1�����ʵķ���ʽΪC4H8O2��������������ĸ��л����ͬ���칹����
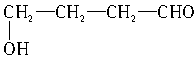 ��
��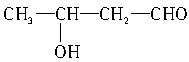 ��
��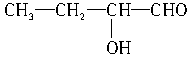 ��
��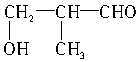 ��
��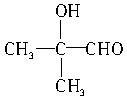 ��5�֡�
��5�֡�
