��Ŀ����
��12�֣�ij�о���ѧϰС�������ֳּ���̽��ǿ��Ͳ�ͬ�����кͷ�Ӧ�Ĺ������£�
(1)ʵ�鲽�裺
�ٷֱ�����Ũ�Ⱦ�Ϊ0.1mol��L-1��NaOH��HC1��CH3 C00H��H3PO4��Һ���á����ƹ������õ��IJ����������ձ�������ƿ����������ϸ��ƿ��____��____��
������ƿ�м���10mL0.1 mol��L-1��HC1����25.00mL____�����ʽ��������ʽ�����ζ����м���0.1 mol��L-1��NaOH���������ݲɼ�����pH��������
������ƿ�е���NaOH���ӽ������NaOH��������ʱ�������μ��ٶȣ��ȶ����ȶ����ٵ���һ��NaOH��
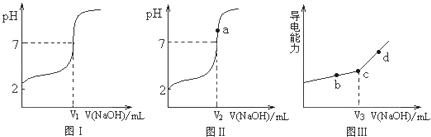
�ܴ洢��������Ƶ�pH�仯ͼ����0.1 mol��L-1��CH3 C00H��H3 P04��Һ����HC1�ظ������ڡ��ܵIJ�����
(2)���������20��ʱNaOH�ֱ�ζ�HC1��CH3 C00H��H3 P04��pH�仯�������¡�
�����������ش��������⣺
��20��ʱ���������ǿ������˳���� ��
�ڵζ���ʼ��������߱仯��������ԭ���� ��
�۴���ǡ���к�ʱpH��8��ԭ���� ��
������Ϊǰ���IJ����У�NaOH�ζ������Ƿ���У� ������С����������С�����
��12�֣�
��1����ͷ�ιܡ���Ͳ ��ʽ
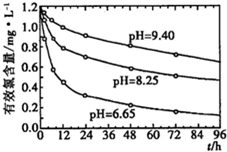
��2�� ������>����>����� ���������ᣬ�кͷ�Ӧ���ɵĴ�������ӶԴ���ĵ���������ƣ����кͺ����ɵĴ�����ˮ��ʹ��Һ�Լ��Ԣܲ�����
����:

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�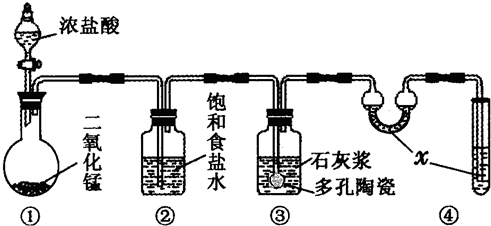


 ��ͼ��ij�о���ѧϰС������ʵ������������װ�������ȡ��ˮ���������ʵ�飮
��ͼ��ij�о���ѧϰС������ʵ������������װ�������ȡ��ˮ���������ʵ�飮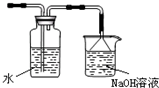 ��
��