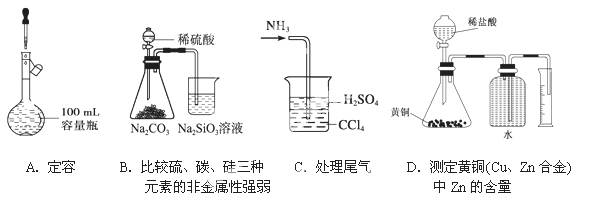
��Ŀ����
���и���ʵ��������������ó��Ľ����У���ȷ����
| ѡ�� | ʵ����������� | ʵ����� |
| A | �����ݵ�������Һ�зֱ�μ�NaCl��Һ�� CuSO4��Һ�����й������� | �����ʾ������˱��� |
| B | ȡ����Fe��NO3��2������ˮ�ܽ⣬��ϡ�����ữ�� �Ρ���KSCN��Һ����Һ��Ϊ��ɫ | ��Fe��NO3��2�����Ѿ� ���� |
| C | ������ij���ʵ���Һ�μӵ����Ƶ�������Һ�У� ˮԡ���Ⱥ����������� | ������һ������ȩ�� |
| D | ͬ�����£��ֱ�0.1mol��L��1������ʹ���� �е�����ʵ�飬����ᴮ���ĵ��ݽϰ� | ���������� |
D
������������� ������Һ�μ�NaCl��Һ���й���������ԭ���Ƿ�������������A�����Fe��NO3��2��NO3?������ϡ�������Һ�ֺ��д���H+������Һ����HNO3, HNO3���Fe��NO3��2����Ϊ Fe��NO3��2������˵��ԭ�������ʣ���B�����������Ҳ�ܷ���������Ӧ���������Dz���ȩ����C�������ͬŨ�ȵ�����ʹ�����ͬ�����½��е�����ʵ�飬����ᴮ���ĵ��ݽϰ���˵���������̶�С��Ϊ���ᣬ��D����ȷ��
���㣺 ���⿼�鵰���ʡ�����������ԡ�������Ӧ���������Һ�ĵ����ԡ�

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�ʵ�������������ʵ����淽������ȷ���ǣ� ��
| A������Һ�����ˮ��棬��ֹ��ӷ� |
| B�����������Ʊ�����ú���� |
| C��Ũ�����ô�������ϸ�ڡ���ɫ�Լ�ƿʢ�ţ��������������� |
| D����������������Һʱ��Ҫ�����м���������������� |
Ϊ�ⶨ̼�����ƴ��ȣ����������Ȼ��ƣ���ijѧ��������ͼʵ��װ�ã�����ʵ�������ȷ����
| A������ϡ�������ϡ���� |
| B����������Һ��Ϊˮ |
| C����Ӧ��ȫ����ȴ����ȡ��������������ټ�ȥ����������������Ϊ���ɵ�������� |
| D��ʵ���ø�������̼��������������ƫ�ߣ������Dzⶨ�������ʱδ��ȴ������ |
���и���ʵ��������������ó��Ľ��۲���ȷ����
| ѡ�� | ʵ����� | ʵ������ | �� �� |
| A | �ⶨ��Ũ�ȵ�Na2CO3��Na2SO3 ��Һ��pH | ǰ��pH�Ⱥ��ߵĴ� | �ǽ����ԣ�S��C |
| B | SO2ͨ�����Ը��������Һ | ���������Һ��ɫ | SO2����Ư���� |
| C | ��������NaOH��Һ���Ⱥ�HNO3�ữ��AgNO3��Һ | ���ֵ���ɫ���� | �����鷢����ˮ�� |
| D | ���Ũ�ȵ�KBr��KI���Һ�еμ�AgNO3��Һ | �ȳ��ֻ�ɫ���� | Ksp(AgBr)> Ksp(AgI) |
ijͬѧ��ʵ�鱨���м�¼�������ݣ�������ȷ���ǣ� ��
| A����25mL��Ͳ��ȡ12.36mL���� |
| B����������ƽ��ȡ8.75gʳ�� |
| C������ʽ�ζ�����ȡ23.22mL���������Һ |
| D���ù㷺pH��ֽ���ij��ҺpHΪ3.5 |
������������ȷ����
| A��ʵ��I :��Ӧ��ʼʱ��Ӧ�����������,�ٱ��� |
| B��ʵ��II :�μӼ���ŨH2SO4��,��Һ��ɫ�ɻ�ɫ��Ϊ��ɫ |
| C��ʵ��III:�ɸ�ʵ�������֤�����Ĵ�Ч�� |
| D��ʵ��IV:����֤AgCl������ת��Ϊ�ܽ�ȸ�С��Ag2S���� |
��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã�Na2SO3 + S  Na2S2O3����������Һ����������ΪNa2S2O3?5H2O��Na2S2O3��5H2O��40��45���ۻ���48��ֽ⣻Na2S2O3������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ����������ͼ��ʾ��
Na2S2O3����������Һ����������ΪNa2S2O3?5H2O��Na2S2O3��5H2O��40��45���ۻ���48��ֽ⣻Na2S2O3������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ����������ͼ��ʾ��
���ְ����·����Ʊ�Na2S2O3��5H2O��
�����ƺ�̼���ư���ӦҪ�����һ������������ƿ�У�ע��150mL����ˮʹ���ܽ⣬�ڷ�Һ©���У�ע��Ũ���ᣬ��װ��2�м����������ƹ��壬������ͼ��װ��װ�á�
��1������2������Ϊ ��
װ��6�пɷ��� ��
| A��BaCl2��Һ | B��ŨH2SO4 | C������KMnO4��Һ | D��NaOH��Һ |
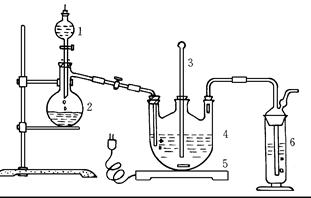
��2����Һ©��������ע��Ũ����ʹ��Ӧ�����Ķ�����������Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У����ô������������������ȣ���Ӧԭ��Ϊ��
��Na2CO3+SO2 =Na2SO3+CO2
��Na2S+SO2+H2O=Na2SO3+H2S
��2H2S+SO2=3S��+2H2O
��Na2SO3+S
 Na2S2O3
Na2S2O3�ܷ�ӦΪ��2Na2S+Na2CO3+4SO2= 3Na2S2O3+CO2
���Ŷ������������ͨ�룬������Һ���д���dz��ɫ��������������ͨ�����������壬��ӦԼ��Сʱ������Һ��pH�ӽ���С��7ʱ������ֹͣͨ���ͼ��ȡ���ҺPHҪ���Ʋ�С��7������
�������ӷ���ʽ��ʾ����
����Na2S2O3��5H2O���궨��Һ��Ũ�ȣ�

��1��Ϊ���ٲ�Ʒ����ʧ��������Ϊ ���������dz���ϴ�Ӹ������ϴ�Ӳ�������
�����Լ�����ϴ�Ӽ���
��2������Ũ����Һֱ����Һ����ɫ����Ϊֹ������ʱΪʲôҪ�����¶Ȳ��˹���
��3����ȡһ�������IJ�Ʒ���ó������������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ��÷�����ƽ
ȷ��ȡ������K2Cr2O7��Ħ������294g/mol��0.5880�ˡ�ƽ���ֳ�3�ݷֱ����3����ƿ�У���
ˮ�����Һ�������������KI���ữ���������з�Ӧ��6I-+Cr2O72-+14H+=3I2+2Cr3++7H2O���ټ��뼸��
������Һ������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-=2I-+S4O62-���ζ��յ������Ϊ
��������Na2S2O3��Һ��ƽ�����Ϊ20.00mL�������궨�������������Һ
��Ũ��Ϊ mol/L��
����ʵ���������ʵ��Ԥ��ʵ��Ŀ�Ļ����ý���һ�µ���( )
| ѡ�� | ʵ���������ʵ | ʵ��Ŀ�Ļ���� |
| A | ij��Һ ð������ ð������ | ˵��ԭ��Һ��һ������CO32- |
| B | SiO2�봿����¿�����CO2 | ˵����������Ա�̼��ǿ |
| C | �⻯����Һ�����Ի�ɫ | ������I-����ԭ��������I2������Һ�� |
| D | ��������Ũ�����н��ݺ���������ˮ��ϴ��Ȼ�����CuSO4��Һ�в���Ӧ | ˵�����������γ���һ�������ȶ�������Ĥ |