��Ŀ����
ij�о���ѧϰС������Na2O2��ˮ��Ӧʵ��ʱ������Na2O2��ˮ��Ӧ�����Һ�еμӷ�̪��Һ��Һ���ֺ�ɫ������ɫ�ܿ���ɫ���ס��ҡ�����λͬѧ�Դ�����ֱ����������Ʋ⣺�ף���Ϊ��Ӧ���Թܺ��ȣ����Կ�������Һ�¶Ƚϸ�ʹ��ɫ��ȥ��
�ң���Ϊ����ˮ�������٣���ɫ��ȥ���������ɵ�NaOH��ҺŨ�Ƚϴ��Ӱ�졣
����Na2O2����ǿ�����ԣ���������O2��H2O2(���ܲ���)��Ҳ����ǿ�����ԣ�����������Ư���˺�ɫ���ʡ�
(1)��֤��ͬѧ���Ʋ��Ƿ���ȷ�ķ�����__________________����֤��ͬѧ���Ʋ��Ƿ���ȷ�ķ�����________________��������________________����ʱ������˵����ͬѧ���Ʋ���ȷ��
(2)��ͬѧ����ö����ķ���̽����ͬѧ�������Ƿ���H2O2����ʵ�鷽��Ϊ����ȡ
�ٲ����������ʱ��������Թܺ���Ͳ�ڵ����嶼��ȴ������ʱ���У�Ӧѡ������ͼװ���е�________��������________________________________��
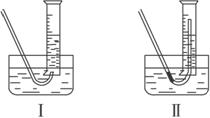
�����ڱ�״���²�������������Ӧѡ�õ���Ͳ�Ĵ�С���Ϊ________(�100 mL����200 mL����500 mL����1 000 mL��)��
(1)��ȴ��ӷ�̪��Һ����ȴ����Һ�Ƿ��� ��ˮϡ�ͺ���Һ�Ƿ���(��ӽ϶��ˮ��Ӧ��μӷ�̪��Һ���Ƿ���) ��ȴ��ˮϡ�ͺ�������
(2)�٢� ��ֹ������ȴʱ������������ ��500 mL

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�ף���Ϊ������NaOH��Һ��Ӧ��ʹ��Һ���Լ������������ԣ�������Һ��ɫ��ȥ���ң���Ϊ��������ˮ����ˮ��Ӧ����HClO������HClO������Ư�����ö�ʹ��Һ��ɫ��?
��1����֤��ͬѧ���Ʋ��Ƿ���ȷ�ķ����ǣ�________________________________��?
��֤��ͬѧ���Ʋ��Ƿ���ȷ�ķ����ǣ�________________________��?
��2�������NaOH��Һ�ij�KMnO4������Һ����Cl2����SO2���壬������ͼʾ����װ�������Ȫʵ���Ƿ����?________(���������������)��?
�����Ϊ����������˵���������________________________________________________________��?
��3�����ơ���ɫ��Ȫʵ�顱���ж��֡���ɫ����Ȫʵ�顱�����㰴ʵ��Ҫ�ֱ����1������ɫ��Ȫʵ�顱����д���б���
��� | ʵ��Ҫ�� | �ձ��е���Һ | �ι��е�Һ�� | ��ƿ�е����� |
�� | ��ɫ���ɫ��Ȫ |
| H2O |
|
�� | ��ɫ����ɫ��Ȫ |
|
| SO2 |

�ף���Ϊ������NaOH��Һ��Ӧ��ʹ��Һ���Լ������������ԣ�������Һ��ɫ��ȥ���ң���Ϊ��������ˮ����ˮ��Ӧ����HClO������HClO������Ư�����ö�ʹ��Һ��ɫ��
(1)��֤��ͬѧ���Ʋ��Ƿ���ȷ�ķ����ǣ�____________________��
��֤��ͬѧ���Ʋ��Ƿ���ȷ�ķ����ǣ�______________________��
(2)�����NaOH��Һ�ij�KMnO4������Һ����Cl2����SO2���壬������ͼʾ����װ�������Ȫʵ���Ƿ����? _____________(���������������)�������Ϊ����������˵���������______________________________��
(3)���ơ���ɫ��Ȫʵ�顱���ж��֡���ɫ����Ȫʵ�顱�����㰴ʵ��Ҫ�ֱ����1������ɫ��Ȫʵ�顱����д���б���
��� | ʵ��Ҫ�� | �ձ��е���Һ | �ι��е�Һ�� | ��ƿ�е����� |
�� | ��ɫ���ɫ��Ȫ |
| H2O |
|
�� | ��ɫ����ɫ��Ȫ |
|
| SO2 |

ͼ1-5-30
�ף���Ϊ������NaOH��Һ��Ӧ��ʹ��Һ���Լ������������ԣ�������Һ��ɫ��ȥ��
�ң���Ϊ��������ˮ����ˮ��Ӧ����HClO������HClO������Ư�����ö�ʹ��Һ��ɫ��
(1)��֤��ͬѧ���Ʋ��Ƿ���ȷ�ķ����ǣ���֤��ͬѧ���Ʋ��Ƿ���ȷ�ķ�����_____________________________________________________________________��
(2)�����NaOH��Һ�ij�����KMnO4��Һ����Cl2����SO2���壬������ͼʾ����װ�������Ȫʵ���Ƿ������__________ (���������������)�������Ϊ����������˵��������ɣ�_____________________________________________________________________��
(3)���ơ���ɫ��Ȫʵ�顱���ж��֡���ɫ����Ȫʵ�顱�����㰴ʵ��Ҫ�ֱ����1������ɫ��Ȫʵ�顱������д���б���
��� | ʵ��Ҫ�� | �ձ��е���Һ | �ι��е�Һ�� | ��ƿ�е����� |
�� | ��ɫ���ɫ����Ȫ |
| H2O |
|
�� | ��ɫ����ɫ����Ȫ |
|
| SO2 |
(4)���������������Ȫʵ���װ��(��ͼ1-5-31��ʾ����ͷ�������㹻���Ľ�����)����ԭ����_______________��ʵ������з��ֿ�ʼʱ�������Ȫѹ����ˮ�����������Խ��Խ����������ƿ���ܳ�����Һ���������㹻���ģ�װ��Ҳ��©������˵��ԭ��________________
____________________________________________________________________��

ͼ1-5-31