��Ŀ����
��1��37Cl�����ӽṹʾ��ͼ ��������ȵ���������SO2��SO3��������ԭ�Ӹ���֮
Ϊ ��
��2��20.6g NaR ����Na+0.2mol����NaR��Ħ������Ϊ ����R 8.0g ��NaR�����ʵ���Ϊ ��
��3���ڱ�״���£�4.8g����(CH4)��ռ�����Ϊ_________L�������״����________L����(H2S)������ͬ��Ŀ����ԭ�ӣ�
��4����100gŨ��Ϊ18 mol��L��1���ܶ�Ϊ��(g��cm�C3)��Ũ�����м���һ������ˮϡ�ͳ�9mol��L��1�����ᣬ�����ˮ����� 100mL�������������������������
��1�� 5: 6
5: 6
��2��103 g/mol����λ��д���÷֣��� 0.1 mol
��3��6.72�� 13.44
��4����
���������������1��Cl?Ϊ18���ӣ������������Ϊ8��������ȵ���������SO2��SO3��������ԭ�Ӹ���֮Ϊm��64��2��m��80��3=5:6��
��2��Na+Ϊ0.2mol����NaRΪ0.2mol��M(NaR)=20.6g��0.2mol=103g/mol����NaR��Ħ�������ɵ�M(R)=80g/mol����n(NaR)=n(R)=8.0g��80g/mol=0.1mol��
��3����������Ħ��������м��㣬V(CH4)=4.8g��16g/mol��22.4L/mol=6.72L��4.8g��16g/mol��4=V(H2S)��22.4L/mol��2,�ɵ�V(H2S)=13.44L��
��4����18 mol��L-1H2SO4ϡ��Ϊ9mol��L-1��Ӧʹ��Һ�����Ϊԭ����2������Ϊ������Һ���ܶ�����Ũ�ȵļ�С����С��ϡ�ͺ��������Һ����С��ԭ��Һ��2�������Լ���ˮ�����С��100ml��
���㣺���⿼�黯ѧ�����ѧ���㡣

ʵ��������Ҫ����500 mL 0.10 mol·L-1��NaOH��Һ����ʵ��ش��������⡣
��1����������ƽ����NaOH��̬ g������NaOH������ע�������������⣺����ΪNaOH���и�ʴ�ԣ����Գ���ʱ��Ӧѡ�� ʢװNaOH���壻�ڳ�������Ѹ�٣�Ŀ���Ƿ�ֹ ��
��2��ʵ������Ҫ��������������ƽ��ҩ���⣬����Ҫ�IJ��������У� �� �� �� ��
��3�����в����������Ƶ���ҺŨ��û��Ӱ����� ��
| A������ʱ���ӿ̶��� |
| B�����ձ����ܽ�����Һ����ע������ƿ��Ȼ������������ˮ���̶��� |
| C��ҡ�ȶ��ݺ��ý�ͷ�ι�������ƿ�еμ�����ˮ���̶��� |
| D��������Һǰ������ˮ��ϴ����ƿ |
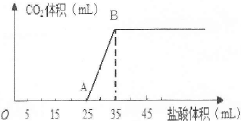
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
| ���� ����ʽ��HCl ��Է���������36.5,�ܶȣ�1.19 g��cm��3 HCl������������36.5% |
��1����Ũ������HCl�����ʵ���Ũ��Ϊ__ ____mol��L��1��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����________��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl������Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.400 mol��L��1��ϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ___ _____mL����Ũ����������ơ�
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿(����������A��ʾ��ƫ����B��ʾ��ƫС������C��ʾ����Ӱ�족)��
a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�� ( )
b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ ( )
��4���ټ����ͬѧ�ɹ�������0.400 mol��L��1�����ᣬ�����ø������кͺ�0.4 g NaOH��NaOH��Һ�����ͬѧ��ȡ________mL���ᡣ����ȷ��С�����һλ��
�ڼ����ͬѧ�������Ƶ������кͺ�0.4 g NaOH��NaOH��Һ�����ֱȢ����������ƫС������ܵ�ԭ����________��
A��Ũ����ӷ���Ũ�Ȳ���
B��������Һʱ��δϴ���ձ�
C��������Һʱ����������ƿ�̶���
D����ˮʱ�����̶��ߣ��ý�ͷ�ι�����