��Ŀ����
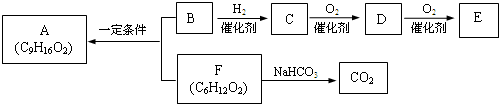
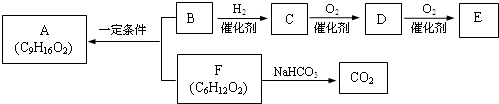
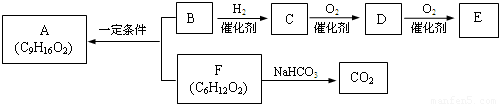
ʳ������A��������֧����B����������B��ʹBr2��CCl4��Һ��ɫ��1molB������Na��Ӧ����0.5molH2�������ʼ�������ת����
�Իش�
��1����һ������������C������Ӧ����______��
a��Na b��Na2CO3 c��Br2/CCl4 d��CH3COOH
��2��D�����еĹ�����������______��E�Ľṹ��ʽ��______��
��3��B��F��Ӧ����A�Ļ�ѧ����ʽ��______��
��4��F��ͬ���칹����ܷ���ˮ�ⷴӦ�����е�һ�ֲ����ܷ���������Ӧ����һ�����ڹ��������µ�һ��ȡ����ֻ�����֣��Ҵ�������IJ���Ҳ�ܷ���������Ӧ��
�Ľṹ��ʽ��______��
��5����֪������C��ͬϵ�������������ڳ�����Ϊ��̬�����ҳ������Ժϳ��л��ܼ��죨C7H14O3��������������2������

��Ľṹ��ʽ��______��

�Իش�
��1����һ������������C������Ӧ����______��
a��Na b��Na2CO3 c��Br2/CCl4 d��CH3COOH
��2��D�����еĹ�����������______��E�Ľṹ��ʽ��______��
��3��B��F��Ӧ����A�Ļ�ѧ����ʽ��______��
��4��F��ͬ���칹����ܷ���ˮ�ⷴӦ�����е�һ�ֲ����ܷ���������Ӧ����һ�����ڹ��������µ�һ��ȡ����ֻ�����֣��Ҵ�������IJ���Ҳ�ܷ���������Ӧ��

�Ľṹ��ʽ��______��
��5����֪������C��ͬϵ�������������ڳ�����Ϊ��̬�����ҳ������Ժϳ��л��ܼ��죨C7H14O3��������������2������

��Ľṹ��ʽ��______��
B��F��Ӧ����A���ɷ���ʽ��ֻB��Ӧ����3��Cԭ�ӣ�B����������B��ʹBr2��CCl4��Һ��ɫ��˵������C=C��1molB������Na��Ӧ����0.5molH2��˵�������к���C=C����BӦΪCH2=CHCH2OH������CΪCH3CH2CH2OH��DΪCH3CH2CHO��EΪCH3CH2COOH��ʳ������A��������֧����B��F����������Ӧ��F����Na2CO3��Ӧ����CO2��Ӧ����-COOH���Ҳ�����֧����ӦΪCH3��CH2��4COOH����AΪCH3��CH2��4COOCH2CH=CH2��
��1�������Ϸ��ӿ�֪CΪCH3CH2CH2OH������-OH���ɷ���������Ӧ����ȥ��Ӧ��������Ӧ�Լ�������Ʒ�Ӧ�����ʣ��ʴ�Ϊ��a��d��
��2��BΪCH2=CHCH2OH������̼̼˫�����ǻ��ȹ����ţ�E�Ľṹ��ʽ��CH3CH2COOH��
�ʴ�Ϊ��̼̼˫�����ǻ��� CH3CH2COOH��
��3��B��F��Ӧ����A�Ļ�ѧ����ʽ��CH3��CH2��4COOH+CH2=CHCH2OH
CH3��CH2��4COOCH2CH=CH2+H2O��
�ʴ�Ϊ��CH3��CH2��4COOH+CH2=CHCH2OH
CH3��CH2��4COOCH2CH=CH2+H2O��
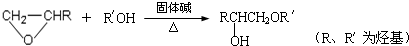
��4��FΪCH3��CH2��4COOH��F��ͬ���칹����ܷ���ˮ�ⷴӦ��Ӧ����-COO-�����е�һ�ֲ����ܷ���������Ӧ��ӦΪ����������һ������ŨH2SO4�������������Ȳ��ܲ���ʹBr2��CCl4��Һ��ɫ���л��˵�����ܷ�����ȥ��Ӧ����-OH��λCԭ���ϲ���Hԭ�ӣ������������IJ���Ҳ�ܷ���������Ӧ��˵������-CH2OH������л���Ľṹ��ʽӦΪ����CH3��3CCH2OOCH���ʴ�Ϊ����CH3��3CCH2OOCH��
��5��CΪCH3CH2CH2OH������C��ͬϵ����ڴ������ʣ�����������Ϊȩ�࣬�ڳ�����Ϊ��̬����Ϊ��ȩ���������Ǽ״���EΪCH3CH2COOH��������ķ���ʽ�����Ƶñ�Ϊ��
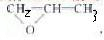
����Ϊ��CH3OCH2CH2OH��������Ľṹ��ʽ��CH3OCH2CH��CH3��OOCCH2CH3���ʴ�Ϊ��CH3OCH2CH��CH3��OOCCH2CH3��
��1�������Ϸ��ӿ�֪CΪCH3CH2CH2OH������-OH���ɷ���������Ӧ����ȥ��Ӧ��������Ӧ�Լ�������Ʒ�Ӧ�����ʣ��ʴ�Ϊ��a��d��
��2��BΪCH2=CHCH2OH������̼̼˫�����ǻ��ȹ����ţ�E�Ľṹ��ʽ��CH3CH2COOH��
�ʴ�Ϊ��̼̼˫�����ǻ��� CH3CH2COOH��
��3��B��F��Ӧ����A�Ļ�ѧ����ʽ��CH3��CH2��4COOH+CH2=CHCH2OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3��CH2��4COOH+CH2=CHCH2OH
| Ũ���� |
| �� |
��4��FΪCH3��CH2��4COOH��F��ͬ���칹����ܷ���ˮ�ⷴӦ��Ӧ����-COO-�����е�һ�ֲ����ܷ���������Ӧ��ӦΪ����������һ������ŨH2SO4�������������Ȳ��ܲ���ʹBr2��CCl4��Һ��ɫ���л��˵�����ܷ�����ȥ��Ӧ����-OH��λCԭ���ϲ���Hԭ�ӣ������������IJ���Ҳ�ܷ���������Ӧ��˵������-CH2OH������л���Ľṹ��ʽӦΪ����CH3��3CCH2OOCH���ʴ�Ϊ����CH3��3CCH2OOCH��
��5��CΪCH3CH2CH2OH������C��ͬϵ����ڴ������ʣ�����������Ϊȩ�࣬�ڳ�����Ϊ��̬����Ϊ��ȩ���������Ǽ״���EΪCH3CH2COOH��������ķ���ʽ�����Ƶñ�Ϊ��

����Ϊ��CH3OCH2CH2OH��������Ľṹ��ʽ��CH3OCH2CH��CH3��OOCCH2CH3���ʴ�Ϊ��CH3OCH2CH��CH3��OOCCH2CH3��

��ϰ��ϵ�д�
�����Ŀ
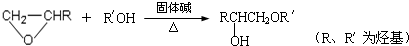


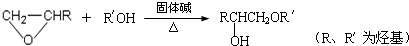

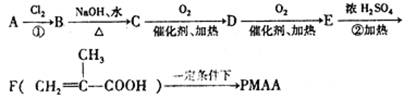
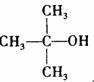 �ṹ���ƵĴ����ܱ�����Ϊȩ��ͪ���������͡��߷��������﹤������ �㷺��Ӧ��ǰ����PMAA����һ�֡������͡��߷��ӣ���Ӧ����������ҩ�д���Ӻ�С���ӵķ��롣������������AΪ��ʼ��Ӧ��ϳ�PMAA��·�ߣ�
�ṹ���ƵĴ����ܱ�����Ϊȩ��ͪ���������͡��߷��������﹤������ �㷺��Ӧ��ǰ����PMAA����һ�֡������͡��߷��ӣ���Ӧ����������ҩ�д���Ӻ�С���ӵķ��롣������������AΪ��ʼ��Ӧ��ϳ�PMAA��·�ߣ�