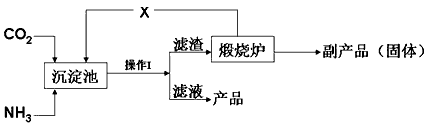
��Ŀ����
I��N2��g��+3H2��g��?2NH3��g����H=-90.0kJ/mol��II.2N2��g��+6H2O��l��?4NH3��g��+3O2��g����H=+1530.0kJ/mol��
��1������������Ӧ��д����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽ
��2���ں��º��ݵļ����������º�ѹ���������зֱ���кϳɰ���Ӧ����ͼ��ͼ����ʾ���ݾ�Ϊ��ʼ����������
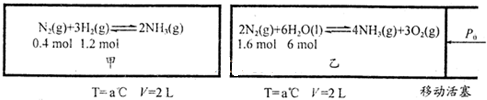
��Ӧ���ﵽƽ��ʱ������NH3Ҳ��Ϊ0.4mol������ˮ��ѹǿ��Ӱ�켰�������ܽ⣩��
�ٸ������¼������е�K=
�ڸ������£��������м�������0.2mol N2���ﵽƽ��ʱN2ת����=
��3���������������������õ�ⷨ��H2O2���Դ˴����ϰ�ˮ��װ������ͼ��
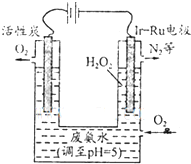
��Ϊ��Ӱ��H2O2�IJ�������Ҫ��ϰ�ˮ�����������������Һ��pHԼΪ5�������÷ϰ�ˮ��Һ��c��NH4+��
��Ir-Ru���Ե缫������O2���ã��õ缫�ķ�ӦΪ
�������ϵ�·��ÿת��3mol���ӣ������Դ���NH3?H2O�����ʵ���Ϊ
��2����ƽ�ⳣ��ָ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ����ѧƽ�ⳣ��ֻ���¶��йأ����ݡ�����ʽ�������м���ó����ۣ��ں��º��ݵ�������ѹǿ֮�ȵ�����������ʵ����ȣ�
�ڸ����¶Ȳ��仯ѧƽ�ⳣ�������ϡ�����ʽ������ó��ﵽƽ��ʱN2ת���ʣ�
��3���ٸ�����Һ�ʵ����ԣ���Һ�����������������������������Һ�������������ĸ�����������н��
���ڸõ����У�Ir-Ru���Ե缫������O2����Ϊ�����õ��ӷ�����ԭ��Ӧ�����������������ɹ������⣻
�۸���4NH3+3O2?2N2+6H2O��ÿת��12mol���ӣ�����4mol�������ݴ˷������
2N2��g��+6H2O��l��?4NH3��g��+3O2��g����H=+1530.0kJ/mol��
�١�
| 2 |
| 3 |
| 1 |
| 3 |
| 2 |
| 3 |
| 1 |
| 2 |
�ʴ�Ϊ��H2��g��+
| 1 |
| 2 |
��2���ٸ������⣺������N2��g��+3H2��g��?2NH3��g����
��ʼ���ʵ�����0.4mol 1.2mol 0
�仯���ʵ�����0.2mol 0.6mol 0.4mol
ƽ�����ʵ�����0.2mol 0.6mol 0.4mol
���Ϊ2L����C��N2��=0.1mol/L��C��H2��=0.3mol/L��C��NH3��=0.2mol/L��ƽ�ⳣ��ΪK=
| C2(NH3) |
| C(N2)��C3(H2) |
| (0.2mol/L)2 |
| 0.1mol/L��(0.3mol/L)3 |
| 400 |
| 27 |
| 1.2 |
| 1.6 |
| 3 |
| 4 |
�ʴ�Ϊ��
| 400 |
| 27 |
| 3 |
| 4 |
�ڻ�ѧƽ�ⳣ��ֻ���¶��йأ���Ϊ���º�ѹ���������⣺������2N2��g��+6H2O��l��?4NH3��g��+3O2��g����
��ʼ���ʵ�����1.6mol 6mol 0 0
�仯���ʵ�����0.2mol 0.6mol 0.4mol 0.3mol
ƽ�����ʵ�����1.4mol 5.4mol 0.4mol 0.3mol
���Ϊ2L����C��N2��=0.7mol/L��C��H2O��=2.7mol/L��C��NH3��=0.2mol/L��C��O2��=0.15mol/L��ƽ�ⳣ��ΪK=
| C3(O2)��C4(NH3) |
| C2(N2)��C6(H2O) |
| (0.3mol )3��(0.4mol )4 |
| (1.4mol)2��(5.4mol )6 |
2N2��g��+6H2O��l��?4NH3��g��+3O2��g����
��ʼ���ʵ�����1.8mol 6mol 0 0
�仯���ʵ�����1.8Xmol 5.4Xmol 3.6Xmol 2.7Xmol
ƽ�����ʵ�������1.8-1.8X��mol�� 6-5.4X��mol 3.6Xmol 2.7Xmol
�¶Ȳ��䣬ƽ�ⳣ�����䣬��
K=
| C3(O2)��C4(NH3) |
| C2(N2)��C6(H2O) |
| (0.3mol )3��(0.4mol )4 |
| (1.4mol)2��(5.4mol )6 |
| (2.7Xmol)3��(3.6Xmol )4 |
| [(1.8-1.8X)mol]2��[( 6-5.4X)mol ]6 |
�ʴ�Ϊ��12.5%��
��3������ϰ�ˮ�����������������Һ��pHԼΪ5��������Һ�ʵ����ԣ���Һ��c��NH4+��+c��H+��=c��NO3-��+c��OH-����pHԼΪ5�����ԣ�c��H+����c��OH-��������
c��NH4+����c��NO3-����
�ʴ�Ϊ������
�����õ�ⷨ��H2O2���ڸõ����У�Ir-Ru���Ե缫������O2����Ϊ�����õ��ӷ�����ԭ��Ӧ��O2+2H++2e-�TH2O2��
�ʴ�Ϊ��O2+2H++2e-�TH2O2��
��4NH3+3O2?2N2+6H2O�У������еĵ�Ԫ�ش�-3�۱�Ϊ�����е�0�ۣ�4mol����ת��12mol���ӣ�����ת��3mol���ӣ������Դ���NH3?H2O�����ʵ���Ϊ1mol��
�ʴ�Ϊ��1mol��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���15�֣����ǵ�ѭ�������е���Ҫ���ʣ����ĺϳ���Ŀǰ�ձ�ʹ�õ��˹��̵�������
��1����֪��H��H����Ϊ436 kJ/mol�� NN����Ϊ945 kJ/mol��N��H����Ϊ391 kJ/mol��д����ҵ�ϳɰ���Ӧ�Ļ�ѧ����ʽ ���ɼ��ܼ���˵���˷�Ӧ�� ��Ӧ������ȡ����ȡ������ϳɰ���Ӧ������1molN2 ʱ���ġ�H = ��
��2�������£���һ��2L���ܱ������г���1 mol N2��2.6 molH2����Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±���ʾ��
| ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
| c��NH3��/��mol/L�� | 0��08 | 0��14 | 0��18 | 0��20 | 0��20 | 0��20 |
5min�ڣ���N2Ũ�ȵı仯��ʾ�ķ�Ӧ����Ϊ ����������, ��Ӧ�ﵽƽ���ʱ��Ϊ�� ���仯ѧƽ�ⳣ��K= ���ﵽƽ�������ת����Ϊ�� ��
��15�֣����ǵ�ѭ�������е���Ҫ���ʣ����ĺϳ���Ŀǰ�ձ�ʹ�õ��˹��̵�������
��1����֪��H��H����Ϊ436 kJ/mol�� N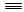 N����Ϊ945 kJ/mol��N��H����Ϊ391 kJ/mol��д����ҵ�ϳɰ���Ӧ�Ļ�ѧ����ʽ
���ɼ��ܼ���˵���˷�Ӧ��
��Ӧ������ȡ����ȡ������ϳɰ���Ӧ������1molN2 ʱ���ġ�H =
��
N����Ϊ945 kJ/mol��N��H����Ϊ391 kJ/mol��д����ҵ�ϳɰ���Ӧ�Ļ�ѧ����ʽ
���ɼ��ܼ���˵���˷�Ӧ��
��Ӧ������ȡ����ȡ������ϳɰ���Ӧ������1molN2 ʱ���ġ�H =
��
��2�������£���һ��2L���ܱ������г���1 mol N2��2.6 mol H2����Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±���ʾ��
|
ʱ��/min |
5 |
10 |
15 |
20 |
25 |
30 |
|
c��NH3��/��mol/L�� |
0��08 |
0��14 |
0��18 |
0��20 |
0��20 |
0��20 |
5min�ڣ���N2Ũ�ȵı仯��ʾ�ķ�Ӧ����Ϊ ����������, ��Ӧ�ﵽƽ���ʱ��Ϊ�� ���仯ѧƽ�ⳣ��K= ���ﵽƽ�������ת����Ϊ�� ��
 ��2010?�ijǶ�ģ��I����ѧ��һֱ�����ڡ��˹��̵������·����о���
��2010?�ijǶ�ģ��I����ѧ��һֱ�����ڡ��˹��̵������·����о���