��Ŀ����
��12�֣��밴ָ��Ҫ������������⡣
�������Ը��������Һ��ͨ������������壬������ر���ԭΪ�����̣�����д���ӷ���ʽ��___________________��
������������������Һ�еμӹ���˫��ˮ������д���ӷ���ʽ��_____________________��
�����廯������Һ��ͨ������ʵ������������������ӷ���ʽ��__________________��
�Ƚ��������������ڹ�����ϡ���ᣨ����д���ӷ���ʽ��__________________________��
���Ҵ��������ữ���ظ������Һ���������ᣬ�ظ���ر���ԭΪ�����������ɻ�ѧ����ʽ��_______________��
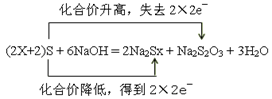
��S + NaOH = Na2Sx + Na2S2O3 + H2O (�ú�x�Ĵ���ʽ��ƽ����ʽ�����������ת�Ƶķ������Ŀ)_________________________________________________________________
�������Ը��������Һ��ͨ������������壬������ر���ԭΪ�����̣�����д���ӷ���ʽ��___________________��
������������������Һ�еμӹ���˫��ˮ������д���ӷ���ʽ��_____________________��
�����廯������Һ��ͨ������ʵ������������������ӷ���ʽ��__________________��
�Ƚ��������������ڹ�����ϡ���ᣨ����д���ӷ���ʽ��__________________________��
���Ҵ��������ữ���ظ������Һ���������ᣬ�ظ���ر���ԭΪ�����������ɻ�ѧ����ʽ��_______________��
��S + NaOH = Na2Sx + Na2S2O3 + H2O (�ú�x�Ĵ���ʽ��ƽ����ʽ�����������ת�Ƶķ������Ŀ)_________________________________________________________________
5SO2 +2MnO4- + 2H2O =2Mn2+ + 5SO42- + 4H+
2Fe2+ + H2O2 +2H+ =2Fe3+ +2H2O 2Fe2+ + 2Br- +2Cl2 = 2Fe3+ + Br2 + 4Cl-
3Fe3O4 + 28H+ +NO3- =9Fe3+ + NO + 14H2O
2K2Cr2O7 + 3C2H5OH +8H2SO4 =2Cr2(SO4)3 + 3CH3COOH + 2K2SO4 +11H2O
2Fe2+ + H2O2 +2H+ =2Fe3+ +2H2O 2Fe2+ + 2Br- +2Cl2 = 2Fe3+ + Br2 + 4Cl-
3Fe3O4 + 28H+ +NO3- =9Fe3+ + NO + 14H2O
2K2Cr2O7 + 3C2H5OH +8H2SO4 =2Cr2(SO4)3 + 3CH3COOH + 2K2SO4 +11H2O

��5SO2 +2MnO4- + 2H2O =2Mn2+ + 5SO42- + 4H+
��2Fe2+ + H2O2 +2H+ =2Fe3+ +2H2O
�� 2Fe2+ + 2Br- +2Cl2 = 2Fe3+ + Br2 + 4Cl-
��3Fe3O4 + 28H+ +NO3- =9Fe3+ + NO + 14H2O
��2K2Cr2O7 + 3C2H5OH +8H2SO4 =2Cr2(SO4)3 + 3CH3COOH + 2K2SO4 +11H2O
��

���鳣����ѧ����ʽ�����ӷ���ʽ����д��ע�������غ㡢����غ㡢���ӵ�ʧ�غ㡣

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ
 2C6H5O- + CO2 ��+ H2O
2C6H5O- + CO2 ��+ H2O