��Ŀ����
����ʵ����ʵ����������Ӧ������ȷ����
| ѡ�� | ʵ����ʵ | ���� |
| A | Na2S2O3��Һ��ϡH2SO4��Һ���ʱ������������ͬ��Na2S2O3��ҺŨ��Խ���������������ʱ��Խ�� | ��������������ʱ������Ӧ��Ũ�Ȼ�ѧ��Ӧ���ʼӿ� |
| B | �ڻ�ѧ��Ӧǰ�����������ͻ�ѧ���ʶ�û�з����ı� | ����һ�����μӻ�ѧ��Ӧ |
| C | ��NH4Cl������Ba(OH)2.8H2O�����Ϻ���ĥ���ձ����¶Ƚ��� | �÷�ӦΪ���ȷ�Ӧ |
| D | ���ݻ��ɱ���ܱ������з�����Ӧ H2��g��+ I2��g��  2HI��g���� 2HI��g�������ݻ���Сһ�� | ����Ӧ���ʼӿ죬�淴Ӧ���ʲ��� |
AC
��ȷ��AC
A����������������ʱ������Ӧ��Ũ�Ȼ�ѧ��Ӧ���ʼӿ졣
B���ڻ�ѧ��Ӧǰ�����������ͻ�ѧ���ʶ�û�з����ı䣬���������ʸı䣬�������ܲμӷ�Ӧ��
C��NH4Cl������Ba(OH)2.8H2O����Ϊ���ȷ�Ӧ��
D��H2��g��+ I2��g��
 2HI��g�������ݻ���Сһ����Ũ�Ⱦ��������淴Ӧ���ʾ��ӿ죬ֻ�DZ仯�ķ�����ͬ������ƽ�ⲻ�ƶ���
2HI��g�������ݻ���Сһ����Ũ�Ⱦ��������淴Ӧ���ʾ��ӿ죬ֻ�DZ仯�ķ�����ͬ������ƽ�ⲻ�ƶ���
��ϰ��ϵ�д�
�����Ŀ


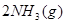 ��H=
��H=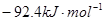 �����¡��������ݻ���ͬ�������ܱ�����A��B��A��ͨ��1molN2��3 molH 2��B ��ͨ��0.5 molN2��1.5 mol H 2����Ӧһ��ʱ���A��B�о��ﵽƽ��״̬�������ж���ȷ���ǣ� ��
�����¡��������ݻ���ͬ�������ܱ�����A��B��A��ͨ��1molN2��3 molH 2��B ��ͨ��0.5 molN2��1.5 mol H 2����Ӧһ��ʱ���A��B�о��ﵽƽ��״̬�������ж���ȷ���ǣ� ��

 �ڹ��պ͵�ȼ������
�ڹ��պ͵�ȼ������ ��ͬ
��ͬ 2CO(g) ��H��0 ����Ӧ����Ϊ u1��N2��3H2
2CO(g) ��H��0 ����Ӧ����Ϊ u1��N2��3H2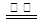 CO2(g)����H��E1 ��
CO2(g)����H��E1 �� _____������ڡ��������ڡ�����С�ڡ���;��II�ų���������
_____������ڡ��������ڡ�����С�ڡ���;��II�ų��������� ��_____________________________________��
��_____________________________________��
 OH)3��+�� ��H2Te��
OH)3��+�� ��H2Te�� 8
8 2NH3��2 min�ﵽƽ��״̬ʱ��H2ת������50%�����¶��µ�ƽ�ⳣ��K��______________(�÷�����ʾ)����ʹK�����Բ�ȡ�Ĵ�ʩ��___________________��
2NH3��2 min�ﵽƽ��״̬ʱ��H2ת������50%�����¶��µ�ƽ�ⳣ��K��______________(�÷�����ʾ)����ʹK�����Բ�ȡ�Ĵ�ʩ��___________________��
 S>0����
S>0����