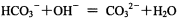��Ŀ����
�������ӷ���ʽ��ȷ����( )
| A��Na2S2O3��Һ�м���ϡ���2S2O32����2H��=SO42����3S����H2O |
B����������������ϡ���3Fe3O4��28H����NO3�� 9Fe3����NO����14H2O 9Fe3����NO����14H2O |
| C��100ml0.1mol/L FeI2��Һ������0.224L Cl2: 2Fe2++ Cl2=2Fe3++2Cl- |
| D����������Һ�еμ�Ba(OH)2��Һ��ǡ��ʹSO42-������ȫ��2Al3++3SO42-+3Ba2++6OH -="2" Al(OH)3��+3BaSO4�� |
B
���������A�����ӷ���ʽû����ƽ��Oԭ�Ӳ��غ㣬����B������������ΪFe3O4����H+��NO3?����������ԭ��Ӧ������Fe3+��NO��H2O����ȷ��C��I?��ԭ�Դ���Fe2+������Cl2��������I?������D����������Һ�еμ�Ba(OH)2��Һ��ǡ��ʹSO42-������ȫ��KAl(SO4)2��Ba(OH)2�����ʵ���֮��Ϊ1:2���������ӷ���ʽ��Al3+��OH?1:4������[Al(OH)4]?������

��ϰ��ϵ�д�
�����Ŀ

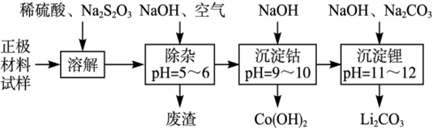
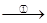 TiCl4
TiCl4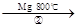 Ti
Ti Fe3+ +3NO2+3H2O
Fe3+ +3NO2+3H2O