��Ŀ����
�����������������õķ���ʴ�ԣ��ڹ�����ҵ���зdz���Ҫ�����á����������գ�
��1����ԭ�Ӻ���������� �ֲ�ͬ����չ������ �ֲ�ͬ�˶�״̬�ĵ��ӡ�
��2���أ�Ga������ͬ�塣д���ص��Ȼ���Ͱ�ˮ��Ӧ�Ļ�ѧ����ʽ��
��3��������ͬ���ڡ�SiO2�ǹ����β�����Na2CaSi6O14������Ҫ�ɷ֣�Na2CaSi6O14Ҳ��д��Na2O��CaO��6SiO2��ʢ��NaOH��Һ���Լ�ƿ���ò���ƿ�������γ�ճ�ԵĹ����ζ�����������Ӧ�Ļ�ѧ����ʽ ��
��ʯ���������ᣬ��ͬ�ʯ����ԭ�ӵ����ʵ���������ͬ�����Ƴ�ʯ��ѧʽNaAlSi3O8����֪�Ƴ�ʯ�Ļ�ѧʽΪ
��4�������ͽ��������ﷴӦ�Ʊ����������ǹ�ҵ�Ͻϳ��õķ������磺
2Al+4BaO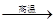 3Ba��+BaO��Al2O3
3Ba��+BaO��Al2O3
������Al�Ľ����Ա�Ba�Ľ����� ��ѡ�ǿ����������������������������ȡBa����Ҫԭ���� ��
a������ʱAl�Ļ����Դ���Ba b������������BaO�ֽ�
c������ʱBaO��Al2O3��Al2O3�ȶ� d��Ba�ķе��Al�ĵ�
��1��4��13��
��2��GaCl3��3NH3+3H2O=3NH4Cl+Ga(OH)3����
��3��SiO2��2NaOH= Na2SiO3��H2O��CaAl2Si2O8��
��4������d
������1����ԭ�Ӻ����������s��p���ֱ���1��3����չ�����������13�����ӣ�����13�ֲ�ͬ�˶�״̬��
��2�������Ȼ���������������Һ��Ӧ��
��3�����ݲ�ͬ�ʯ����ԭ�ӵ����ʵ���������ͬ����ϻ��ϼ۴�������0��д���Ƴ�ʯ�Ļ�ѧʽ��
��4���÷�Ӧ������Ba�ķе��Al�ĵͣ��������ݳ���ʹƽ�����ơ�
�����㶨λ�����⿼��Al��Ga��Si���仯����Ľṹ���ʵȡ�
