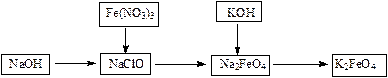��Ŀ����
�����ĺ��ϵ����滷�����̲��ŷḻ�ĺ�ˮ��Դ�������йغ�ˮ�ۺ����õ�˵����ȷ���ǣ� ��
| A���Ӻ�ˮ�пɵõ�NaCl���������NaCl����ˮ��Һ�����Ƶ�Cl2 |
| B����ˮ�к���þԪ�أ��ʲ��辭����ѧ�仯�Ϳ��Եõ�þ���� |
| C����������ԭ���Ӻ�ˮ����ȡ��ˮ�Ǻ�ˮ����������չ���·��� |
| D�������Ǻ�ˮ���˷��绹�dz�ϫ���磬���ǽ���ѧ��ת��Ϊ���� |
A
��

��ϰ��ϵ�д�
�����Ŀ