��Ŀ����
A��B��C��D��E��ԭ��������������Ķ�����Ԫ�أ�A��D�����ڱ��е����λ�����±�����Ԫ��A����������ϼ���������ϼ۵ľ���ֵ���2��B��D����ͬ����Ԫ�أ�Ԫ��C��һ������ɫ�����������ڿ����л�Ѹ�ٱ������ɰ�ɫ���ʡ�

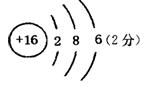
��1��D��ԭ�ӽṹʾ��ͼΪ ��
��2��Ԫ��E��Ԫ�����ڱ���λ�ڵ� �壻
��3��C��D��E���Ӱ뾶�Ĵ�С��ϵΪ �������ӷ��ű�ʾ����
��4��Ԫ��B�ĵ�����Ԫ��C�ĵ��ʿɷ�����ѧ��Ӧ���ɻ�����ף���Ļ�ѧʽΪ
��ʵ��֤�����ܹ���ˮ������ѧ��Ӧ����д������ˮ��Ӧ�����ӷ���ʽ
��
��5��������Ԫ��A�������̬�⻯�����Ԫ��C������������Ӧ��ˮ���
��pH��ͬ���ұ���ˮ��Һ���ֱ�������ˮϡ�͵�ԭ����x��y����ϡ�ͺ�������Һ��pH��Ȼ��ͬ����x y����д����������������=������
�������ӹ�ҵ�У��ҵ�ˮ��Һ��������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ ������Ҫ��ƽ����
(1) 
��2����A��1�֣�
��3��Cl-��S2-��Na+��2�֣�
(4��Na2O��Na2O2(2�֣�
Na2O+H2O=2Na++2OH-��2Na2O2+2H2O=4Na++4OH-+O2��(4��)
��5���٣���2�֣� ��NH��H2O+H2O2��N2��+H2O��2�֣�
����:
��

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�| A�������ӵİ뾶��C��D��E��B | B����ҵ�ϳ��õ�ⷨ�Ƶ�C��D�ĵ��� | C���ȶ��ԣ�A2B��A2E | D������D������ұ��ijЩ���۽��� |

 2DB3����3.2gDB2��ȫת��ΪDB3����ʱ����akJ��1mol DB3������ȫת��Ϊ��ˮ�������bkJ��������33.6L DB2��ȫ�������������Ӧ����
2DB3����3.2gDB2��ȫת��ΪDB3����ʱ����akJ��1mol DB3������ȫת��Ϊ��ˮ�������bkJ��������33.6L DB2��ȫ�������������Ӧ����
