��Ŀ����
��12�֣���X��Y��Z��W�Ƕ����ڵ�����Ԫ�أ��й����ǵ���Ϣ���±���ʾ��
| Ԫ�� | ���ֽṹ֪ʶ | �������� |
| X | X�ĵ�����˫ԭ�ӷ��ӹ��ɣ���������14������ | X�ж����������XO��XO2��X2O4�ȣ�ͨ�������XO2��X2O4���� |
| Y | Yԭ�ӵĴ�������������������������һ�� | Y���γɶ�����̬������ |
| Z | Zԭ�ӵ���������������4 | ZԪ�ص���������ϼ���������ϼ۴����͵���6 |
| W | Wԭ�ӵ���������������2n-3��nΪԭ�Ӻ�����Ӳ����� | ��ѧ��Ӧ��Wԭ����ʧȥ���������γ�Wn+ |
��д���пհף�����ʾ����������ĸX��Y��Z��W������
��1��д��X���ʵ�һ����Ҫ��; ����������
��2��X��Y��Z��Ԫ�ص�����������ˮ����������ǿ������˳���� ���ѧʽ����
��3��ʵ�����Ʊ�X�����̬�⻯��Ļ�ѧ����ʽ
��4��������Y�����������ͨ���������Һ�з�Ӧ�����ӷ���ʽ
��5��W����������������Һ��Ӧ�����ӷ���ʽ
��6��Z�����ڼ��ȵ���������Ũ����������Һ��Ӧ�����������뻹ԭ��������ʵ���֮
��Ϊ1:5��Ӧ�����ӷ���ʽ
��������X�ĵ�����˫ԭ�ӷ��ӹ��ɣ���������14�����ӣ�����X��ԭ��������7����ΪN����������������������������һ���ԭ����C����Y��C����������ϼ���������ϼ۴����͵���6��˵��Z���ڵ� ��A����ΪFû�����ۣ�����Z��Cl����������������2n-3��nΪԭ�Ӻ�����Ӳ���������nֻ����2��3������ΪWԭ����ʧȥ���������γ�Wn+������n����3����W��Al�����������������Ƶķ�Ӧ�У���ԭ�����������ӣ�����������ͻ�ԭ��������ʵ���֮����1�U5����������������Ԫ�صĻ��ϼ��ǣ�5�ۡ���ΪClO3����
��1���ϳɰ���ԭ���� ��2��HClO4 >HNO3>H2CO3���ѧʽ����
��3�� 2NH4Cl+Ca(OH)2 CaCl2+2NH3����2H2O
��4��CO2+SiO32��+H2O=CO32��+H2SiO3��
��5��2Al+2OH��+2H2O=2AlO2��+3H2��
��6��3Cl2+6OH��=5Cl��+ClO3��+3H2O

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�| A��XԪ�ص��⻯���ˮ��Һ�Լ��� | B��ZԪ�ص����Ӱ뾶����WԪ�ص����Ӱ뾶 | C��ZԪ�صĵ�����һ������������XԪ�صĵ��ʷ�Ӧ | D��YԪ�����������ľ�����кܸߵ��۵�ͷе� |
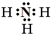
 Al��OH��3+3H+
Al��OH��3+3H+