��Ŀ����
��֪��A��B��C��D��E��F����ѧ��ѧ���������ֳ��������ʣ�����֮������ͼ��ʾ���ת����ϵ����Ӧ���������ֲ���δ�������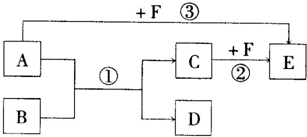
��ش��������⣺
��1����A��D�������ǵ��ճ�����ϢϢ��ص����ֽ������ʣ�F��һ��ǿ���Ӧ�۵����ӷ���ʽ��______��
����D�����ڳ�ʪ�������ױ�ɺ���ɫ��ĩB����д����Ӧ�ٵĻ�ѧ����ʽ______��
��2����A��B��D�����л������A��B�Ǽ�ͥ�����г��õ�ζƷ����Ҫ�ɷ֣���B����Է���������A��14��E���ܶ���С�����壬��FΪ______�������ƣ�����Ӧ�ٵĻ�ѧ����ʽΪ______��
�⣺��1����A��D�������ǵ��ճ�����ϢϢ��ص����ֽ������ʣ�F��һ��ǿ���A��ǿ�Ӧ��֪AΪ����Al������D�����ڳ�ʪ�������ױ�ɺ���ɫ��ĩB��BΪFe2O3��DΪFe����A+B��C+D��֪��CΪAl2O3���ɷ�Ӧ��Al2O3 E����Ӧ��Al
E����Ӧ��Al E��֪��EΪƫ�����Σ�
E��֪��EΪƫ�����Σ�
��Ӧ��������ǿ�Ӧ����ƫ����������������Ӧ���ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
��Ӧ���������������ڸ��µ������·�Ӧ������������������Ӧ����ʽΪ��2Al+Fe2O3 2Fe+Al2O3��
2Fe+Al2O3��
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����2Al+Fe2O3 2Fe+Al2O3��
2Fe+Al2O3��
��2����A��B��D�����л������A��B�Ǽ�ͥ�����г��õ�ζƷ����Ҫ�ɷ֣���B����Է���������A��14��AΪC2H5OH��BΪCH3COOH��E���ܶ���С�����壬EΪH2����ת����ϵ��A+B��C+D��֪��DΪCH3COOCH2CH3��CΪH2O���ɷ�Ӧ��H2O H2����Ӧ��C2H5OH
H2����Ӧ��C2H5OH H2��֪��FΪNa����Ӧ�ٵĻ�ѧ����ʽΪ��C2H5OH+CH3COOH
H2��֪��FΪNa����Ӧ�ٵĻ�ѧ����ʽΪ��C2H5OH+CH3COOH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
�ʴ�Ϊ���ƣ�C2H5OH+CH3COOH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��������1����A��D�������ǵ��ճ�����ϢϢ��ص����ֽ������ʣ�F��һ��ǿ���A��ǿ�Ӧ��֪AΪ����Al������D�����ڳ�ʪ�������ױ�ɺ���ɫ��ĩB��BΪFe2O3��DΪFe����A+B��C+D��֪��CΪAl2O3���ɷ�Ӧ��Al2O3 E����Ӧ��Al
E����Ӧ��Al E��֪��EΪƫ�����Σ�
E��֪��EΪƫ�����Σ�
��2����A��B��D�����л������A��B�Ǽ�ͥ�����г��õ�ζƷ����Ҫ�ɷ֣���B����Է���������A��14��AΪC2H5OH��BΪCH3COOH��E���ܶ���С�����壬EΪH2����ת����ϵ��A+B��C+D��֪��DΪCH3COOCH2CH3��CΪH2O���ɷ�Ӧ��H2O H2����Ӧ��C2H5OH
H2����Ӧ��C2H5OH H2��֪��FΪNa��
H2��֪��FΪNa��
�����������Կ�ͼ�����ʽ����Al��Fe��Ԫ�ص��ʼ��仯����֮����ת����ϵ��������������ʡ���ѧ�������д�ȣ��ѶȲ���ע�����֪ʶ���������գ�
 E����Ӧ��Al
E����Ӧ��Al E��֪��EΪƫ�����Σ�
E��֪��EΪƫ�����Σ���Ӧ��������ǿ�Ӧ����ƫ����������������Ӧ���ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
��Ӧ���������������ڸ��µ������·�Ӧ������������������Ӧ����ʽΪ��2Al+Fe2O3
 2Fe+Al2O3��
2Fe+Al2O3���ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����2Al+Fe2O3
 2Fe+Al2O3��
2Fe+Al2O3����2����A��B��D�����л������A��B�Ǽ�ͥ�����г��õ�ζƷ����Ҫ�ɷ֣���B����Է���������A��14��AΪC2H5OH��BΪCH3COOH��E���ܶ���С�����壬EΪH2����ת����ϵ��A+B��C+D��֪��DΪCH3COOCH2CH3��CΪH2O���ɷ�Ӧ��H2O
 H2����Ӧ��C2H5OH
H2����Ӧ��C2H5OH H2��֪��FΪNa����Ӧ�ٵĻ�ѧ����ʽΪ��C2H5OH+CH3COOH
H2��֪��FΪNa����Ӧ�ٵĻ�ѧ����ʽΪ��C2H5OH+CH3COOH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O���ʴ�Ϊ���ƣ�C2H5OH+CH3COOH
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O����������1����A��D�������ǵ��ճ�����ϢϢ��ص����ֽ������ʣ�F��һ��ǿ���A��ǿ�Ӧ��֪AΪ����Al������D�����ڳ�ʪ�������ױ�ɺ���ɫ��ĩB��BΪFe2O3��DΪFe����A+B��C+D��֪��CΪAl2O3���ɷ�Ӧ��Al2O3
 E����Ӧ��Al
E����Ӧ��Al E��֪��EΪƫ�����Σ�
E��֪��EΪƫ�����Σ���2����A��B��D�����л������A��B�Ǽ�ͥ�����г��õ�ζƷ����Ҫ�ɷ֣���B����Է���������A��14��AΪC2H5OH��BΪCH3COOH��E���ܶ���С�����壬EΪH2����ת����ϵ��A+B��C+D��֪��DΪCH3COOCH2CH3��CΪH2O���ɷ�Ӧ��H2O
 H2����Ӧ��C2H5OH
H2����Ӧ��C2H5OH H2��֪��FΪNa��
H2��֪��FΪNa�������������Կ�ͼ�����ʽ����Al��Fe��Ԫ�ص��ʼ��仯����֮����ת����ϵ��������������ʡ���ѧ�������д�ȣ��ѶȲ���ע�����֪ʶ���������գ�

��ϰ��ϵ�д�
�����Ŀ
 ��2011?�����ģ����ѧ--ѡ�����ʽṹ������
��2011?�����ģ����ѧ--ѡ�����ʽṹ������
 ��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
 ��֪����A��B��C��D�����µķ�Ӧ��ϵ������A ��B��ȼ��ʱ������ʲ�ɫ�� C��B��ȼ��ʱ�����ػ�ɫ���̣�E��ˮ��Һ������ɫ��G��һ�ֺ�ɫ���壮
��֪����A��B��C��D�����µķ�Ӧ��ϵ������A ��B��ȼ��ʱ������ʲ�ɫ�� C��B��ȼ��ʱ�����ػ�ɫ���̣�E��ˮ��Һ������ɫ��G��һ�ֺ�ɫ���壮