��Ŀ����
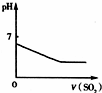
��2011?�Ͳ���ģ����1����20.00mLϡ��ˮ����μ�������ʵ���Ũ�ȵ����ᣬ��ش�������⣺
�ٵ�����10.00mL����ʱ����Һ�и�������Ũ��֮��Ĵ�С��ϵΪ�������������������ӷ��ţ���c��
�ڵ������백ˮ�����ʵ�����Ӧʱ����Һ��pH
�۵���ҺpH=7ʱ����Ӧ�����ĵ����ʣ�����������Һ�д��������ʣ�֮��Ĺ�ϵΪ��n��NH3?H2O��
��2����֪ij�������ʽ�������HB-���ܷ������룬���ܷ���ˮ�⣬��ͨ��ʵ��ȷ��HB-�����ǵ���̶ȴ���ˮ��̶ȴ������ʵ����̺ͽ��ۣ�
�ٵ�����10.00mL����ʱ����Һ�и�������Ũ��֮��Ĵ�С��ϵΪ�������������������ӷ��ţ���c��
NH4+
NH4+
����c��Cl-
Cl-
����c��OH-
OH-
����c��H+
H+
���ڵ������백ˮ�����ʵ�����Ӧʱ����Һ��pH
��
��
7�������������=������ͬ���۵���ҺpH=7ʱ����Ӧ�����ĵ����ʣ�����������Һ�д��������ʣ�֮��Ĺ�ϵΪ��n��NH3?H2O��
��
��
n��HCl������Һ��c��NH4+��=
=
c��Cl-������2����֪ij�������ʽ�������HB-���ܷ������룬���ܷ���ˮ�⣬��ͨ��ʵ��ȷ��HB-�����ǵ���̶ȴ���ˮ��̶ȴ������ʵ����̺ͽ��ۣ�
��pH��ֽ��NaHB��Һ��pHֵ��
��pH��ֽ��NaHB��Һ��pHֵ��
��pH��7��˵��HB-ˮ��̶ȴ��������̶ȣ���pH=7��˵����ˮ��̶ȵ��������̶ȣ����pH��7����˵����ˮ��̶�С�������̶ȣ�
��pH��7��˵��HB-ˮ��̶ȴ��������̶ȣ���pH=7��˵����ˮ��̶ȵ��������̶ȣ����pH��7����˵����ˮ��̶�С�������̶ȣ�
����������1�����жϷ�Ӧ����Һ�е����ʣ��ٸ��ݵ���������ˮ����
�������백ˮǡ�÷�Ӧ��Ϊ�Ȼ����Һ����������ˮ������ж���Һ����ԣ�
�����õ���غ��жϳ�c��NH4+�� ��c��Cl-����С��ϵ���ٸ�����Һ�д���ƽ�� NH4++H2O?NH3?H2O�жϣ�
��2�������������ʽ������ӣ�������̶ȴ���ˮ��̶ȣ���Һ�����ԣ�������̶�С��ˮ��̶ȣ�����Һ�ʼ��ԣ�������̶ȵ���ˮ��̶ȣ���Һ�����ԣ��ݴ˽��
�������백ˮǡ�÷�Ӧ��Ϊ�Ȼ����Һ����������ˮ������ж���Һ����ԣ�
�����õ���غ��жϳ�c��NH4+�� ��c��Cl-����С��ϵ���ٸ�����Һ�д���ƽ�� NH4++H2O?NH3?H2O�жϣ�
��2�������������ʽ������ӣ�������̶ȴ���ˮ��̶ȣ���Һ�����ԣ�������̶�С��ˮ��̶ȣ�����Һ�ʼ��ԣ�������̶ȵ���ˮ��̶ȣ���Һ�����ԣ��ݴ˽��
����⣺��1���ٵ�����10.00mL�������ҺΪ�����ʵ���Ũ�ȵİ�ˮ���Ȼ�淋Ļ��Һ����ˮ����ʹ�ʼ��ԣ��Ȼ��ˮ��ʹ��Һ�����ԣ������ʵ���Ũ�ȣ�����̶ȴ���ˮ��̶ȣ���Һ�ʼ��ԣ�����c��OH-����c��H+����
���ݵ���غ��У�c��NH4+��+c��H+��=c��Cl-��+c��OH-������Һ�ʼ��ԣ����ԣ�c��NH4+����c��Cl-����
ˮ��̶�������Һ��c��Cl-��ԶԶ����c��OH-����
����Һ�и�������Ũ��֮��Ĵ�С��ϵΪc��NH4+����c��Cl-����c��OH-����c��H+����
�ʴ�Ϊ��NH4+��Cl-��OH-��H+
�������백ˮǡ�÷�Ӧ��Ϊ�Ȼ����Һ���Ȼ����ǿ�������Σ�����Һ�����ԣ���PH��7
�ʴ�Ϊ����
�۸��ݵ���غ㣺c��NH4+��+c��H+��=c��Cl-��+c��OH-����������ҺpH=7����c��OH-��=c��H+��������c��NH4+����c��Cl-������Һ�д���ƽ�� NH4++H2O?NH3?H2O�����������غ��У���Ӧ�����ĵ����ʣ�����������Һ�д��������ʣ�֮��Ĺ�ϵΪ��n��NH3?H2O����n��HCl����
�ʴ�Ϊ������=
��2����pH��ֽ��NaHB��Һ��pHֵ����pH��7��˵��HB-ˮ��̶ȴ��������̶ȣ���pH=7��˵����ˮ��̶ȵ��������̶ȣ����pH��7����˵����ˮ��̶�С�������̶ȣ�
�ʴ�Ϊ����pH��ֽ��NaHB��Һ��pH����pH��7��˵��HB-ˮ��̶ȴ��������̶ȣ���pH=7��˵����ˮ��̶ȵ��������̶ȣ����pH��7����˵����ˮ��̶�С�������̶ȣ�
���ݵ���غ��У�c��NH4+��+c��H+��=c��Cl-��+c��OH-������Һ�ʼ��ԣ����ԣ�c��NH4+����c��Cl-����
ˮ��̶�������Һ��c��Cl-��ԶԶ����c��OH-����
����Һ�и�������Ũ��֮��Ĵ�С��ϵΪc��NH4+����c��Cl-����c��OH-����c��H+����
�ʴ�Ϊ��NH4+��Cl-��OH-��H+
�������백ˮǡ�÷�Ӧ��Ϊ�Ȼ����Һ���Ȼ����ǿ�������Σ�����Һ�����ԣ���PH��7
�ʴ�Ϊ����
�۸��ݵ���غ㣺c��NH4+��+c��H+��=c��Cl-��+c��OH-����������ҺpH=7����c��OH-��=c��H+��������c��NH4+����c��Cl-������Һ�д���ƽ�� NH4++H2O?NH3?H2O�����������غ��У���Ӧ�����ĵ����ʣ�����������Һ�д��������ʣ�֮��Ĺ�ϵΪ��n��NH3?H2O����n��HCl����
�ʴ�Ϊ������=
��2����pH��ֽ��NaHB��Һ��pHֵ����pH��7��˵��HB-ˮ��̶ȴ��������̶ȣ���pH=7��˵����ˮ��̶ȵ��������̶ȣ����pH��7����˵����ˮ��̶�С�������̶ȣ�
�ʴ�Ϊ����pH��ֽ��NaHB��Һ��pH����pH��7��˵��HB-ˮ��̶ȴ��������̶ȣ���pH=7��˵����ˮ��̶ȵ��������̶ȣ����pH��7����˵����ˮ��̶�С�������̶ȣ�
����������Ũ�ȴ�С�Ƚϵĵ�ʽ��ϵ����Ҫ���ݡ������غ㡱��������غ㡢�����غ�������غ㣮

��ϰ��ϵ�д�
 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
�����Ŀ