��Ŀ����
|
��֪�Ȼ�ѧ����ʽ��2SO2(g)��O2(g) | |
| [����] | |
A�� |
��ͬ�����£�2 mol��SO2(g)��1 mol��O2(g)�����е�����С��2 mol��SO3(g)�����е����� |
B�� |
��2 mol��SO2(g)��1 mol��O2(g)����һ�ܱ������г�ַ�Ӧ�ų�����ΪQ kJ |
C�� |
����ѹǿ�������¶ȣ���ƽ�ⶼ���淴Ӧ�����ƶ� |
D�� |
�罫һ����SO2(g)��O2(g)����ij�ܱ������г�ַ�Ӧ����Q kJ����˹�������2 mol��SO2(g)������ |
�𰸣�D

��ϰ��ϵ�д�
�����Ŀ
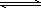 2SO3(g)����H����Q kJ��mol��1(Q��0)������˵����ȷ����
2SO3(g)����H����Q kJ��mol��1(Q��0)������˵����ȷ���� ��ӦC��s��+H2O��g��?CO��g��+H2��g����һ�ݻ��ɱ���ܱ������н��У��Իش�
��ӦC��s��+H2O��g��?CO��g��+H2��g����һ�ݻ��ɱ���ܱ������н��У��Իش�