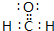��Ŀ����
���ſ�ѧ�����ķ�չ�������ӵ������IJⶨ�ֶ�Խ��Խ�࣬�ⶨ����ҲԽ��Խ�ߣ�����һ�ּ��еIJⶨ���������岽��Ϊ��?�ٽ�����NaCl��ϸ�������ȷ��ȡm g NaCl����ת�Ƶ���������A�У�?
���õζ�����A�����еμӱ����������������ӱ���A�����Ŀ̶��ߣ������NaCl��������ΪV cm3��
(1)�������A���������__________��(����������)?
(2)�����������ʽ�ζ��ܺû����ü�ʽ�ζ��ܺ�__________��������__________________
________________________________��
(3)�ܷ��ý�ͷ�ιܴ��沽����еĵζ���__________��������_________________________
_________________________��
(4)�ܷ���ˮ���汽__________��������________________________________________
_________________________��
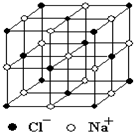
(5)��֪NaCl����ṹ��ͼ3��27��ʾ����X���߲��NaCl�����п��������Na+��Cl-���ƽ������Ϊa cm�����������ⶨ������õİ����ӵ�����NA�ı���ʽΪNA=__________��
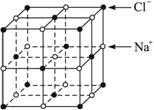
ͼ3��27
����������һ�������ⶨ���⣬��ʵ��ľ��ȿ���A����Ӧѡ������ƿ��������ƿ�мӱ�ʱ�����ڱ����������͡��ϻ����ã����Եζ�������ʽ�ζ��ܣ����ڽ�ͷ�ιܵ��������ý�ͷ�ιܴ���ζ��ܣ�����ˮ���汽��NaCl���ܽ⣬���NaCl���������ȷ�ⶨ��������ͼ�ɿ��������ӶԸýṹ��Ԫ�Ĺ���Ϊ1+12��1/4=4����Na+=6��1/2+8��1/8=4�����ýṹ��Ԫ����4��NaCl���ýṹ��Ԫ�ı߳�Ϊ
�𰸣���1������ƿ?
��2����ʽ�ζ��ܡ����ڱ����������͡��ϻ����ã����Եζ�������ʽ�ζ���?
��3�����ܡ����ڽ�ͷ�ιܵ�����?
��4�����ܡ�����ˮ���汽��NaCl���ܽ⣬���NaCl���������ȷ�ⶨ����?
��5��58.5V/2ma3

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A������ | B��ˮ�� | C�������մ� | D���մ� |
 ���ſ�ѧ�����ķ�չ���ⶨ�����ӵ��������ֶ�Խ��Խ�࣬�ⶨ�ľ�ȷ��ҲԽ��Խ�ߣ�����һ�ּ��еIJⶨ���������������������£�
���ſ�ѧ�����ķ�չ���ⶨ�����ӵ��������ֶ�Խ��Խ�࣬�ⶨ�ľ�ȷ��ҲԽ��Խ�ߣ�����һ�ּ��еIJⶨ���������������������£�