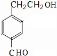
��Ŀ����
(9��)��1����֪25�桢101kPaʱ��һЩ���ʵ�ȼ����Ϊ��
| ��ѧʽ | CO(g) | H2(g) | CH3OH(l) |
| ��H/( kJ��mol��1) | ��283.0 | ��285.8 | ��726.5 |
��ش��������⣺�ٸ�������CH3OH(l)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��__________________________��
�ڸ��ݸ�˹����������з�Ӧ���Ȼ�ѧ����ʽ��CO(g)+2H2(g)===CH3OH(l)��H=____________��
��2���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ�
�� CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H1=+49.0 kJ��mol-1
�� CH3OH(g)+O2(g)=CO2(g)+2H2(g) ��H2
��֪H2(g)+ O2(g)===H2O(g) ��H = -241.8 kJ��mol-1
��Ӧ�ڵġ�H2 = kJ��mol-1��
��3���״�ȼ�ϵ�صĽṹʾ��ͼ���¡��״����� ��������������������������ĵ缫��ӦΪ ��
��1����CH3OH(l)+![]() O2(g)===CO2(g)+2H2O(l) ��H=��726.5 kJ��mol��1 �� 2�֣�
O2(g)===CO2(g)+2H2O(l) ��H=��726.5 kJ��mol��1 �� 2�֣�
�ڣ�128.1 kJ��mol��1 ��2�֣�
��2��-192.8 ��2�֣�
��3������1�֣� O2 + 4H+ + 4e��=== 2H2O ��2�֣�
����:��1��ȼ������ָ��һ�������£���ȼ����ȫȼ�������ȶ���������ʱ���ų������������Ը�������CH3OH(l)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��CH3OH(l)+![]() O2(g)===CO2(g)+2H2O(l) ��H=��726.5 kJ��mol��1 ����ΪCO������ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ
O2(g)===CO2(g)+2H2O(l) ��H=��726.5 kJ��mol��1 ����ΪCO������ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ
��CO��g)+ O2(g)===CO2(g) ��H=��283kJ��mol��1 ����2H2��g����O2(g)=2H2O(l) ��H=��571.6 kJ��mol��1 �����ݸ�˹���ɢڣ��ۣ��ٿɵ�CO(g)+2H2(g)===CH3OH(l) ��H=��128.1 kJ��mol��1 ��
O2(g)===CO2(g) ��H=��283kJ��mol��1 ����2H2��g����O2(g)=2H2O(l) ��H=��571.6 kJ��mol��1 �����ݸ�˹���ɢڣ��ۣ��ٿɵ�CO(g)+2H2(g)===CH3OH(l) ��H=��128.1 kJ��mol��1 ��
��2����Ȼ�����ڸ�˹���ɽ��м��㡣����ӦH2(g)+ O2(g)===H2O(g) ��H = -241.8 kJ��mol-1�ͷ�ӦCH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H1=+49.0 kJ��mol-1�ϲ����ɡ�
��3���״�ʧȥ���ӣ�ͨ�븺��������ͨ�������������缫��ӦʽΪO2 + 4H+ +4e��=== 2H2O ��

 ��У����ϵ�д�
��У����ϵ�д�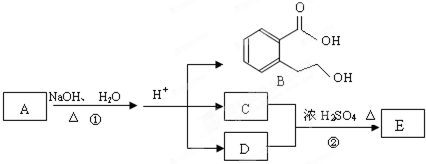

 ����1��
����1��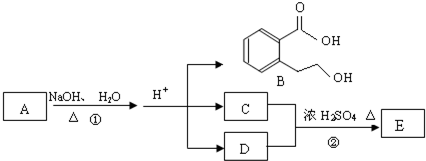

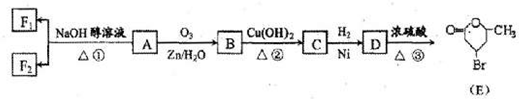
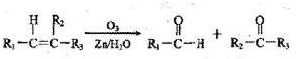
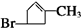


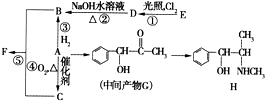 �л���A����Ҫ�Ļ����ϳ�ԭ�ϣ���ҽҩ��Ⱦ�Ϻ����ϵ���ҵ���Ź㷺��Ӧ�ã���A�Ƶ�ijҩ��H��ת����ϵ��ͼ��ʾ��A��G��G��H�ķ�Ӧ�����Ͳ��ַ�Ӧ������ȥ����
�л���A����Ҫ�Ļ����ϳ�ԭ�ϣ���ҽҩ��Ⱦ�Ϻ����ϵ���ҵ���Ź㷺��Ӧ�ã���A�Ƶ�ijҩ��H��ת����ϵ��ͼ��ʾ��A��G��G��H�ķ�Ӧ�����Ͳ��ַ�Ӧ������ȥ����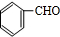
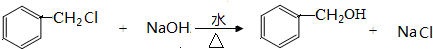
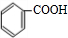 +
+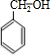
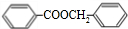 +H2O
+H2O