��Ŀ����
ij��ȤС���������ͼʵ��װ�ý���ʵ�顣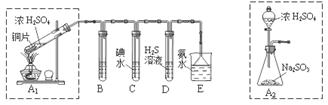
��̽��������Ⱦ��SO2������
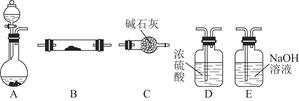
��1��Ϊ��ʵ����ɫ������Ŀ�꣬�ܷ�����ͼA2����A1װ�� ����ܡ�����
��2��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ ��C�з�Ӧ�����ӷ���ʽΪ ��D�з�Ӧ�Ļ�ѧ����ʽΪ ��
��̽��ͭƬ��ŨH2SO4��Ӧ�IJ���
ʵ�������������ͭƬ���渽�ź�ɫ���塣�������ϵ�֪���˺�ɫ������ܺ���CuO��CuS��Cu2S��������CuS��Cu2S��������ϡ���ᣬ�ڿ��������ն�ת��ΪCu2O��SO2����С��ͬѧ�ռ�һ������ɫ���壬������ʵ�鷽��̽����ɷ֣�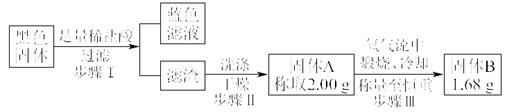
��3������� �м�������ϴ�Ӹɾ���ʵ�鷽����_____________________________��
��4����ɫ����ijɷ���________________��
�������
�ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH����SO32����SO42����HSO3���������ӡ�
��5����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽΪ ��
��6����֪����������һ��������ˮ��SO2Ҳ������ˮ��
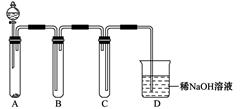
�����������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2 mol/L���ᡢ
2 mol/LHNO3��1 mol/LBaCl2��Һ��l mol/LBa(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32����HSO3��������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡl mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ�������Һ�� �д���SO32���� SO42���� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� �� | �� ������Һ���д��� SO32���� |
| ����3�� �� | �� ������Һ���д��� HSO3���� |
��1���ܣ�1�֣�
��2��Ʒ����Һ��1�֣� SO2��I2��2H2O��SO42����2 I����4H+��2�֣�
SO2��2H2S="3S��+" 2H2O��2�֣�
��3��ȡ���һ��ϴ��Һ�������μ�����AgNO3��Һ����������������ϴ�Ӹɾ���2�֣���
��4��CuO��CuS��Cu2S��2�֣�
��5��NH3��H2O��SO2��NH4+��HSO3����2�֣�
��6������1�֣�ʵ����� Ԥ����������� ����2������1�Σ���������Ʒ����Һ���ٵ������2mol/L���ᣬ�� ��Ʒ����ɫ���������ݡ�����������ܽ⣩�� ����3�����Թ�ȡ������Һ�������е��������1mol/LBa(OH)2��Һ [�����1�Σ���������Ʒ����Һ���ٵ���2-3�Σ����������2mol/L����]���� �����ְ�ɫ��������Ʒ����Һ��ɫ���������ݣ�
���������������B��ʢ��Ʒ�죬�ܼ���SO2��Ư���ԡ����ˮ����SO2��I2��2H2O��SO42����2 I����4H+֤���仹ԭ�ԡ�D�з�ӦSO2��2H2S="3S��+" 2H2O��֤���������ԣ�
��ȡ���һ��ϴ��Һ�������μ�����AgNO3��Һ����������������ϴ�Ӹɾ�������1�����������ɫ��Һ��ȷ����CuO,�ڿ��������ն�ת��ΪCu2O��SO2, ��Ӧ����ʽΪ��4CuS+3O2=2Cu2O+4SO2
��2Cu2S+3O2=2Cu2O+2SO2����2g��������AΪCuS,��Ϸ���ʽ�ټ���ù�������BΪ1.47g�������������AΪCu2S����Ϸ���ʽ�ڼ���ù�������B������Ϊ1.8g������B������Ϊ1.68g������ͬʱ����CuS��Cu2S��
��ˮ���չ�����SO2�����ӷ���ʽΪNH3��H2O��SO2��NH4+��HSO3-֤��SO32���Ĵ��ڣ�������������м������ᣬ��Ʒ������Ƿ����SO2��֤��HSO3-��������ȿ����ᷴӦ��Ҳ����Ӧ�����ʡ�
���㣺��Ԫ�ػ�������صĻ�ѧʵ�顣

��Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᡣ
��1�������й�ʵ�������ʵ����ʵ����������ȷ���� (�����)��
A��ʵ������Ũ����Ӧ��������ɫϸ��ƿ�У���������ͼ��ʾ��ǩ |
| B����50mL��Ͳ��ȡ5��6mLŨ���� |
| C���к͵ζ�ʵ��ʱ����ƿϴ�Ӹɾ����ñ�Һ��ϴ����ע�����Һ |
| D�������Ȼ�̼��ȡ��ˮ�еĵ⣬��Һʱ�л���ӷ�Һ©�����¶˷ų� |
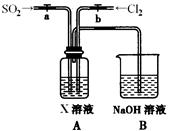
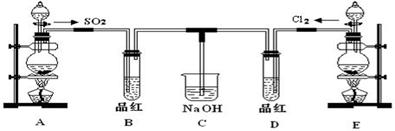
��2����ͼ��ʵ�����Ʊ�������̽�������Ƿ����Ư���Ե�ʵ��װ��(�гּ�����������ʡ��)��
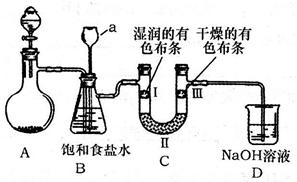
��Aװ���з�Ӧ�Ļ�ѧ����ʽΪ ��
��Bװ��������a�������� ��
��Bװ�õ������dz�ȥ�����л��е�HCl������ȫƿ�����ã�������a��Һ�治
������ʱ��˵�� ����ʱӦֹͣʵ�顣
��ʵ���й۲쵽 ��˵������������Ư���ԡ�
ij��ȤС���������ͼʵ��װ�ý���ʵ�顣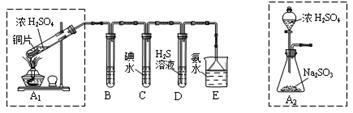
��̽��������Ⱦ��SO2������
��1��Ϊ��ʵ����ɫ������Ŀ�꣬�ܷ�����ͼA2����A1װ�� ����ܡ�����
��2��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ ��C�з�Ӧ�����ӷ���ʽΪ ��D�з�Ӧ�Ļ�ѧ����ʽΪ ��
��̽��ͭƬ��ŨH2SO4��Ӧ�IJ���
ʵ�������������ͭƬ���渽�ź�ɫ���塣�������ϵ�֪���˺�ɫ������ܺ���CuO��CuS��Cu2S��������CuS��Cu2S��������ϡ���ᣬ�ڿ��������ն�ת��ΪCu2O��SO2����С��ͬѧ�ռ�һ������ɫ���壬������ʵ�鷽��̽����ɷ֣�
��3��������м�������ϴ�Ӹɾ���ʵ�鷽����_____________________________��
��4����ɫ����ijɷ���________________��
�������
�ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH-��SO32-��SO42-��HSO3-�������ӡ�
��5����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽΪ ��
��6����֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2 mol/L���ᡢ2 mol/LHNO3��1 mol/LBaCl2��Һ��l mol/LBa(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32-��HSO3-������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡl mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ� ������Һ���д���SO32-�� SO42-�� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� �� | �� ������Һ���д��� SO32-�� |
| ����3�� �� | �� ������Һ���д��� HSO3-�� |
�ף��������о���ѧϰС��Ϊ�ⶨ�������е�����ԭ�Ӹ����ȣ����������ʵ�����̣�
��ͼA��B��CΪ�ף�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��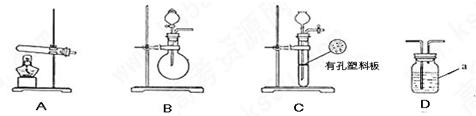
ʵ�鿪ʼǰװ���еĿ������ž�����С���ã���Ӧǰ����ͭ������Ϊ ������ͭ��Ӧ��ʣ����������Ϊ
������ͭ��Ӧ��ʣ����������Ϊ �����ɵ����ڱ�״���µ����
�����ɵ����ڱ�״���µ���� ����С����ϴ��װ��Dǰ������������ɵ����ڱ�״���µ������
����С����ϴ��װ��Dǰ������������ɵ����ڱ�״���µ������
��1��д������a�����ƣ� ��
��2���ף�����С��ѡ���˲�ͬ������ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��ո��С�
| | ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� |
| ��С�� | A | �������ƣ������ | ��Ӧ�Ļ�ѧ����ʽΪ �� |
| ��С�� | �� | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� �� |
��3����С�����������ݼ�����������е������ԭ�Ӹ���֮��Ϊ ��
��4���ڲ����ͼ�����ȷ������£���С�����������ݼ�����������е������ԭ�Ӹ���������С������ֵ����ԭ������� ��
��5����С����ԭ��ʵ��Ļ�����������һ��װ��ҩƷ��ʵ������������ʵ�飬�ó�������ʵ��������ҩƷ�������� ��
 MnCl2+Cl2��+2H2O������6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ ��
MnCl2+Cl2��+2H2O������6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ ��
 ������N2ʱ����Ҫ�İ�������ʯ�Һ�Ũ��ˮ��ԭ����ȡ�����˲������������е�____________����A��B��C������ͬ����NH3�ķ�������Ҫ��ȡ���ռ�N2������ѡ�õ���������___________��
������N2ʱ����Ҫ�İ�������ʯ�Һ�Ũ��ˮ��ԭ����ȡ�����˲������������е�____________����A��B��C������ͬ����NH3�ķ�������Ҫ��ȡ���ռ�N2������ѡ�õ���������___________��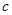 ������ȡN2��������һ�����к����������� ______________________
������ȡN2��������һ�����к����������� ______________________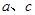 �������ʹ��Խ��Խ�ܵ����ǵĹ�ע�����ַ�����
�������ʹ��Խ��Խ�ܵ����ǵĹ�ע�����ַ�����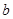 ������ȣ�����Խ������____________��
������ȣ�����Խ������____________��