��Ŀ����
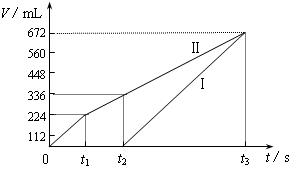
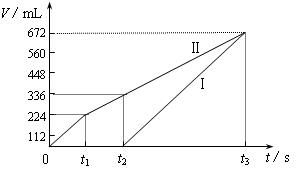
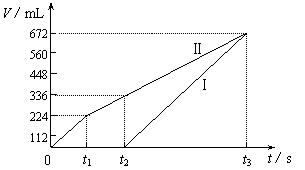
�������ö��Ե缫���200 mL һ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ��������������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⡣
 |
��ͨ�������Ʋ⣺
��ԭ�����ҺNaCl��CuSO4�����ʵ���Ũ�ȣ�
��t2ʱ������Һ��pH��
�����ö��Ե缫���NaCl��CuSO4�Ļ����Һ200mL������һ��ʱ����������õ�224mL���壬��ԭ�����Һ�е�������Ũ�ȵ�ȡֵ��ΧΪ ��ͭ����Ũ�ȵ�ȡֵ��ΧΪ ��
�Ţ����������ݳ�����Cl2
n(NaCl)��2n(Cl2)��0.02 mol����c(NaCl)��0.1mol/L ��1�֣�
�����õ�336 mL�����У���0.01 mol Cl2��0.005 mol O2
ת�Ƶ��ӵ����ʵ���Ϊ��0.01 mol��2��0.005 mol��4��0.04 mol
�˹����������պ�ȫ������ͭ
n(CuSO4)��n(Cu)��0.04 mol��2��0.02 mol
��c(CuSO4)��![]() ��0.1mol/L ��2�֣�
��0.1mol/L ��2�֣�
��t2ʱ��Һ��c(Na��)��0.1 mol/L��c(SO42��)��0.1 mol/L
���ݵ���غ��У�c(H��)��2��0.1 mol/L��0.1 mol/L��0.1 mol/L
����Һ��pH��1 ��2�֣�
��0��c(Cl��)��0.1mol/L��2�֣� 0��c(Cu2��)��0.05mol/L��2�֣�