��Ŀ����
�����£�N2H4���͵����ᶼ�ǵ�Ԫ�ص���Ҫ�⻯�
��1�������������쵪�ʡ�����ȣ�
�ٺϳɰ���ҵ�У����������йط�Ӧ���Ȼ�ѧ����ʽ���£�
C��s��+H2O��g��=CO��g��+H2��g����H1=+131.4kJ?mol-1
C��s��+2H2O��g��=CO2��g��+2H2��G����H2=+90.2kJ?mol-1
CO��g��+H2O��g��=CO2��g��+H2��g����H3
���H3= ��
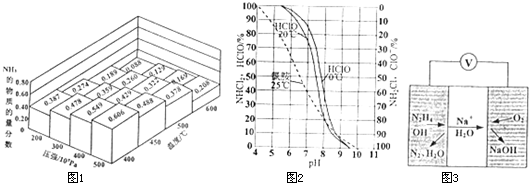
����һ�ܱ������У��������ʵ���֮��Ϊ1��3��N2��H2���ڲ�ͬ�¶ȡ�ѹǿ�²��ƽ����ϵ��NH3�����ʵ���������ͼ1��ʾ�����¶�Ϊ400�桢ѹǿΪ500��105 Paʱ��H2��ƽ��ת������ӽ� ������ţ���
A.89% B.75%
C.49% D.34%
��ʵ�������У��ϳɰ����¶�һ�������400��500�棬ѡ����¶ȷ�Χ�������� ��
������ˮ�Ȼ�����ʱ��ˮ�к��а��������Ȱ���NH2Cl��NHCl2�ȣ����Ȱ�ˮ����ͷų�HClO�������õ�����Ч����ͼ2������ˮ�Ȼ�����ʱ�йسɷֵĺ�����pH�Ĺ�ϵ������˵����ȷ���� ������ţ���
A��HCIO��20��ĵ���̶�һ������0��ĵ���̶�
B��pHԽ������Ч��Խ��
C����NH2Cl����ʱ��ƽ��NH2Cl+H2O?NH3+HClO�����ƶ�
��2���¿����ڻ��ȼ�ϡ���ҩԭ�ϵȣ�
�ٴ�������������İ���Ӧ�����Ʊ��£��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��һ����ȼ�ϵ�صĹ���ԭ����ͼ3��ʾ���õ�ع���ʱ�����ĵ缫��ӦʽΪ ��
�����������ᣨHNO2����Ӧ�����ɵ����ᣮ8.6g��������ȫ�ֽ�ɷų�6.72L��������״���£����������ķ���ʽΪ ��

��1�������������쵪�ʡ�����ȣ�
�ٺϳɰ���ҵ�У����������йط�Ӧ���Ȼ�ѧ����ʽ���£�
C��s��+H2O��g��=CO��g��+H2��g����H1=+131.4kJ?mol-1
C��s��+2H2O��g��=CO2��g��+2H2��G����H2=+90.2kJ?mol-1
CO��g��+H2O��g��=CO2��g��+H2��g����H3
���H3=
����һ�ܱ������У��������ʵ���֮��Ϊ1��3��N2��H2���ڲ�ͬ�¶ȡ�ѹǿ�²��ƽ����ϵ��NH3�����ʵ���������ͼ1��ʾ�����¶�Ϊ400�桢ѹǿΪ500��105 Paʱ��H2��ƽ��ת������ӽ�
A.89% B.75%
C.49% D.34%
��ʵ�������У��ϳɰ����¶�һ�������400��500�棬ѡ����¶ȷ�Χ��������
������ˮ�Ȼ�����ʱ��ˮ�к��а��������Ȱ���NH2Cl��NHCl2�ȣ����Ȱ�ˮ����ͷų�HClO�������õ�����Ч����ͼ2������ˮ�Ȼ�����ʱ�йسɷֵĺ�����pH�Ĺ�ϵ������˵����ȷ����
A��HCIO��20��ĵ���̶�һ������0��ĵ���̶�
B��pHԽ������Ч��Խ��
C����NH2Cl����ʱ��ƽ��NH2Cl+H2O?NH3+HClO�����ƶ�
��2���¿����ڻ��ȼ�ϡ���ҩԭ�ϵȣ�
�ٴ�������������İ���Ӧ�����Ʊ��£��÷�Ӧ�Ļ�ѧ����ʽΪ
��һ����ȼ�ϵ�صĹ���ԭ����ͼ3��ʾ���õ�ع���ʱ�����ĵ缫��ӦʽΪ
�����������ᣨHNO2����Ӧ�����ɵ����ᣮ8.6g��������ȫ�ֽ�ɷų�6.72L��������״���£����������ķ���ʽΪ
��������1���ٸ��ݸ�˹���ɼ��㷴Ӧ�ȣ�
�ڴ�ͼ���ҳ��������µİ���������������������ʽ���м��㣻
��ѡ�����˵��¶ȣ����Ϸ�Ӧ���ʺ��Ƚϴ�
��A����ͼ��ɿ���������ĵ�������¶ȵĹ�ϵ��
B����ͼ��ɿ���pH�������Ũ�ȵĹ�ϵ��
C���������Ϣ�ó����ۣ�
��2���ٴ�������������İ���Ӧ�Ʊ��£����ݷ�Ӧ�����������ԭ���غ�д����ѧ����ʽ��
���ڼ��Ե��������ʧȥ�������ɵ�����ˮ��
�۸������ݼ����N��H�ĸ����ȣ�����д����ѧʽ��
�ڴ�ͼ���ҳ��������µİ���������������������ʽ���м��㣻
��ѡ�����˵��¶ȣ����Ϸ�Ӧ���ʺ��Ƚϴ�
��A����ͼ��ɿ���������ĵ�������¶ȵĹ�ϵ��
B����ͼ��ɿ���pH�������Ũ�ȵĹ�ϵ��
C���������Ϣ�ó����ۣ�
��2���ٴ�������������İ���Ӧ�Ʊ��£����ݷ�Ӧ�����������ԭ���غ�д����ѧ����ʽ��
���ڼ��Ե��������ʧȥ�������ɵ�����ˮ��
�۸������ݼ����N��H�ĸ����ȣ�����д����ѧʽ��
����⣺��1����C��s��+H2O��g��=CO��g��+H2��g����H1=+131.4kJ?mol-1
��C��s��+2H2O��g��=CO2��g��+2H2��G����H2=+90.2kJ?mol-1
��Ӧ�ڼ�ȥ��Ӧ�ٵ÷�Ӧ��CO��g��+H2O��g��=CO2��g��+H2��g����H3
���ԣ���H3=��H2-��H1=90.2kJ?mol-1-131.4kJ?mol-1=-41��kJ?mol-1
�ʴ�Ϊ��-41.2kJ?mol-1��
�ڴ�ͼ�пɿ��������¶�Ϊ400�桢ѹǿΪ500��105 Paʱ�����������ʵ�������Ϊ0.606
��������ת����Ϊx����
N2 +3H2 =2NH3
��ʼ 1 3 0
ת�� x 3x 2x
ƽ�� 1-x 3-3x 2x
=0.606
��ã�x��0.75��
�ʴ�Ϊ��B��
�ۺϳɰ���Ӧ�Ƿ��ȷ�Ӧ���¶�̫�ߣ�NH3���ʵ����������ͣ��¶�̫�ͣ���Ӧ����̫����
��400-500���¶ȷ�Χ�ڣ�����������ߣ�Ϊ���Ϸ�Ӧ���ʺ��Ƚϴ����Ļ��Դ�Ҫѡ�����˵��¶�400-500�棻
�ʴ�Ϊ���¶�̫�ߣ�NH3���ʵ����������ͣ��¶�̫�ͣ���Ӧ����̫�������¶ȷ�Χ�ڣ�����������ߣ�
��A����ͼ��ɿ�������20��ʱ������ĺ�������0�������ĺ�������20��ʱ������ĵ���С��0�������ĵ��룬��A����
B����ͼ��ɿ���pH�������Ũ�ȵĹ�ϵ��pHԽ�������Ũ��ԽС����������Ч��Խ���B����
C���������Ϣ��֪���Ȱ�ˮ����ͷų�HClO�������õ�����Ч��������ƽ�����ƣ���C��ȷ��
��ѡC��
��2���ٴ�������������İ���Ӧ�Ʊ��£�����������Ϊ�£���������ԭΪ�����ӣ�
���ԭ���غ���ƽд����ѧ����ʽΪ��NaClO+2NH3�TN2H4+NaCl+H2O��
�ʴ�Ϊ��NaClO+2NH3�TN2H4+NaCl+H2O��
���ڼ��Ե��������ʧȥ�������ɵ�����ˮ���缫��ӦΪN2H4-4e-+4OH-=N2+4H2O��
�ʴ�Ϊ��N2H4-4e-+4OH-=N2+4H2O��
��8.6g��������ȫ�ֽ�ɷų�6.72L��������֪8.6g�������к�N�����ʵ���Ϊ��
��2=0.6mol��
��H�����ʵ���Ϊ��
=0.2mol��n��N����n��H��=0.6��0.2=3��1�����Ե�����ķ���ʽΪHN3��
�ʴ�Ϊ��HN3��
��C��s��+2H2O��g��=CO2��g��+2H2��G����H2=+90.2kJ?mol-1
��Ӧ�ڼ�ȥ��Ӧ�ٵ÷�Ӧ��CO��g��+H2O��g��=CO2��g��+H2��g����H3
���ԣ���H3=��H2-��H1=90.2kJ?mol-1-131.4kJ?mol-1=-41��kJ?mol-1
�ʴ�Ϊ��-41.2kJ?mol-1��
�ڴ�ͼ�пɿ��������¶�Ϊ400�桢ѹǿΪ500��105 Paʱ�����������ʵ�������Ϊ0.606
��������ת����Ϊx����
N2 +3H2 =2NH3
��ʼ 1 3 0
ת�� x 3x 2x
ƽ�� 1-x 3-3x 2x
| 2x |
| (1-x)+(3-3x)+2x |
��ã�x��0.75��
�ʴ�Ϊ��B��
�ۺϳɰ���Ӧ�Ƿ��ȷ�Ӧ���¶�̫�ߣ�NH3���ʵ����������ͣ��¶�̫�ͣ���Ӧ����̫����
��400-500���¶ȷ�Χ�ڣ�����������ߣ�Ϊ���Ϸ�Ӧ���ʺ��Ƚϴ����Ļ��Դ�Ҫѡ�����˵��¶�400-500�棻
�ʴ�Ϊ���¶�̫�ߣ�NH3���ʵ����������ͣ��¶�̫�ͣ���Ӧ����̫�������¶ȷ�Χ�ڣ�����������ߣ�
��A����ͼ��ɿ�������20��ʱ������ĺ�������0�������ĺ�������20��ʱ������ĵ���С��0�������ĵ��룬��A����
B����ͼ��ɿ���pH�������Ũ�ȵĹ�ϵ��pHԽ�������Ũ��ԽС����������Ч��Խ���B����
C���������Ϣ��֪���Ȱ�ˮ����ͷų�HClO�������õ�����Ч��������ƽ�����ƣ���C��ȷ��
��ѡC��
��2���ٴ�������������İ���Ӧ�Ʊ��£�����������Ϊ�£���������ԭΪ�����ӣ�
���ԭ���غ���ƽд����ѧ����ʽΪ��NaClO+2NH3�TN2H4+NaCl+H2O��
�ʴ�Ϊ��NaClO+2NH3�TN2H4+NaCl+H2O��
���ڼ��Ե��������ʧȥ�������ɵ�����ˮ���缫��ӦΪN2H4-4e-+4OH-=N2+4H2O��
�ʴ�Ϊ��N2H4-4e-+4OH-=N2+4H2O��
��8.6g��������ȫ�ֽ�ɷų�6.72L��������֪8.6g�������к�N�����ʵ���Ϊ��
| 6.72L |
| 22.4L/mol |
��H�����ʵ���Ϊ��
| 8.6g-0.6mol��14g/mol |
| 1g/mol |
�ʴ�Ϊ��HN3��
���������⿼���˸�˹���ɵļ��㡢��Ӧ������ѡ��ƽ��ļ��㼰����ʽ���缫��Ӧʽ����д���ƶϷ���ʽ���Ѷ����У���������ͼ���϶࣬Ҫ��ȷ��������ͼ����Ϣ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
�����Ŀ
 ��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
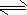 N2H5++OH-
N2H5++OH-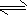 N2H62++OH-
N2H62++OH-  N2H4+H3O+
N2H4+H3O+