��Ŀ����
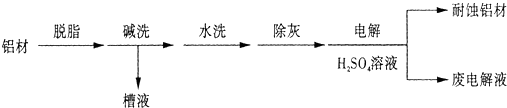
һ�ֺ�����ﮡ��ܵ����͵��Ӳ��ϣ������в����ķ��������ɹۣ������е����Խ�����������ʽ���ڣ�����Co2O3?CoO����ʽ���ڣ������������ĵ����˫�棻﮻��������У��ӷ����л��������ܣ�CoO���Ĺ���������ͼ1��ʾ��
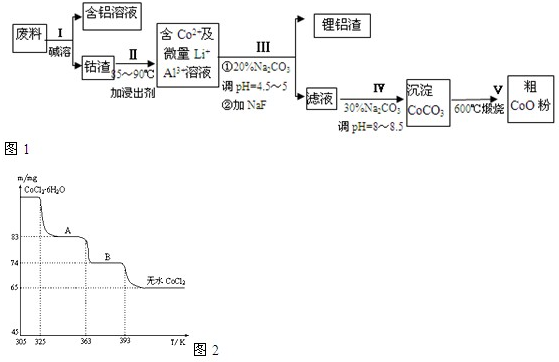
��1�����̢��в���NaOH��Һ�ܳ������е�Al����Ӧ�����ӷ���ʽΪ______��
��2�����̢��м���ϡH2SO4�ữ���ټ���Na2S2O3��Һ�����ܣ�������ܵĻ�ѧ��Ӧ����ʽΪ��������ֻ��һ�������______����ʵ����ģ�ҵ����ʱ��Ҳ������������ܣ���ʵ�ʹ�ҵ�����в������ᣬ��ӷ�Ӧԭ������������������ܵ���Ҫԭ��______��
��3�����̢�õ����������Ҫ�ɷ���LiF��Al��OH��3��̼������Һ�ڲ���Al��OH��3ʱ����Ҫ���ã���д���÷�Ӧ�����ӷ���ʽ______��
��4��̼������Һ�ڹ��̢�͢�����������������ͬ����д���ڹ��̢������������______��
��5����Na2CO3��Һ�д��ڶ������ӣ����и�����Ũ�ȹ�ϵ��ȷ����______������ţ���
A��c��Na+��=2c��CO32-��
B��c��Na+����c��CO32-����c��HCO3-��
C��c��OH-����c��HCO3-����c��H+��
D��c��OH-��-c��H+���Tc��HCO3-��+2c��H2CO3��
��6��CoO��������ɵ÷ۺ�ɫ��CoCl2��Һ��CoCl2���ᾧˮ��Ŀ��ͬ�����ֲ�ͬ��ɫ��������ɫ����ˮCoCl2��ˮ��ɫ��һ���ʿ��Ƴɱ�ɫˮ�������īˮ����ͼ�Ƿۺ�ɫ��CoCl2?6H2O�������ȷֽ�ʱ��ʣ������������¶ȱ仯�����ߣ�A���ʵĻ�ѧʽ��______��

��1�����̢��в���NaOH��Һ�ܳ������е�Al����Ӧ�����ӷ���ʽΪ______��
��2�����̢��м���ϡH2SO4�ữ���ټ���Na2S2O3��Һ�����ܣ�������ܵĻ�ѧ��Ӧ����ʽΪ��������ֻ��һ�������______����ʵ����ģ�ҵ����ʱ��Ҳ������������ܣ���ʵ�ʹ�ҵ�����в������ᣬ��ӷ�Ӧԭ������������������ܵ���Ҫԭ��______��
��3�����̢�õ����������Ҫ�ɷ���LiF��Al��OH��3��̼������Һ�ڲ���Al��OH��3ʱ����Ҫ���ã���д���÷�Ӧ�����ӷ���ʽ______��
��4��̼������Һ�ڹ��̢�͢�����������������ͬ����д���ڹ��̢������������______��
��5����Na2CO3��Һ�д��ڶ������ӣ����и�����Ũ�ȹ�ϵ��ȷ����______������ţ���
A��c��Na+��=2c��CO32-��
B��c��Na+����c��CO32-����c��HCO3-��
C��c��OH-����c��HCO3-����c��H+��
D��c��OH-��-c��H+���Tc��HCO3-��+2c��H2CO3��
��6��CoO��������ɵ÷ۺ�ɫ��CoCl2��Һ��CoCl2���ᾧˮ��Ŀ��ͬ�����ֲ�ͬ��ɫ��������ɫ����ˮCoCl2��ˮ��ɫ��һ���ʿ��Ƴɱ�ɫˮ�������īˮ����ͼ�Ƿۺ�ɫ��CoCl2?6H2O�������ȷֽ�ʱ��ʣ������������¶ȱ仯�����ߣ�A���ʵĻ�ѧʽ��______��
��1����������������Һ��Ӧ����ƫ�����ƺ���������Ӧ�����ӷ�Ӧ����ʽΪ��2Al+2OH-+2H2O=+2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=+2AlO2-+3H2����
��2��Co3O4��Na2S2O3�����������·���������ԭ��Ӧ����CoSO4��Na2SO4��H2O����Ӧ����ʽΪ��4Co3O4+Na2S2O3+11H2SO4=12CoSO4+Na2SO4+11H2O��������л�ԭ�ԣ��ܱ�Co2O3?CoO���������ж�����������Ⱦ���������Բ������ᣬ
�ʴ�Ϊ��4Co3O4+Na2S2O3+11H2SO4=12CoSO4+Na2SO4+11H2O��Co2O3?CoO�������������Cl2��Ⱦ������
��3������������̼������ӷ���˫ˮ���������������Ͷ�����̼��ˮ������ӷ���ʽΪ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����
�ʴ�Ϊ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����
��4��̼������Һ�ڹ��̢�������������̼������ӷ���˫ˮ���������������Ͷ�����̼��̼������Һ�ڹ��̢��е���pH���ṩCO32-��ʹCo2+����ΪCoCO3��
�ʴ�Ϊ������pH���ṩCO32-��ʹCo2+����ΪCoCO3��
��5��A��Na2CO3��Һ�е���غ㣺c��Na+��+c��H+��=c��HCO3-��+c��OH-��+2c��CO32-������A����
B��̼������ӷ���ˮ���Լ�ˮ�ĵ��룬������Һ������Ũ�ȣ�c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+������B��ȷ��
C��̼������ӷ���ˮ���Լ�ˮ�ĵ��룬������Һ������Ũ�ȣ�c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+������C��ȷ��
D��Na2CO3��Һ�������غ㣺c��H+���Tc��OH-��+c��HCO3-��+2c��H2CO3������D��ȷ��
��ѡ��BCD��
��6��CoCl2?6H2O��CoCl2
238 130
m65mg
=
��ã�m=119mg
A���ʵĻ�ѧʽΪCoCl2?nH2O�����У�
CoCl2?6H2O��CoCl2?nH2O��m
238 18��6-n��
119mg119mg-83mg
=
����ã�n=2��
����A���ʵĻ�ѧʽΪ��CoCl2?2H2O���ʴ�Ϊ��CoCl2?2H2O��
�ʴ�Ϊ��2Al+2OH-+2H2O=+2AlO2-+3H2����
��2��Co3O4��Na2S2O3�����������·���������ԭ��Ӧ����CoSO4��Na2SO4��H2O����Ӧ����ʽΪ��4Co3O4+Na2S2O3+11H2SO4=12CoSO4+Na2SO4+11H2O��������л�ԭ�ԣ��ܱ�Co2O3?CoO���������ж�����������Ⱦ���������Բ������ᣬ
�ʴ�Ϊ��4Co3O4+Na2S2O3+11H2SO4=12CoSO4+Na2SO4+11H2O��Co2O3?CoO�������������Cl2��Ⱦ������
��3������������̼������ӷ���˫ˮ���������������Ͷ�����̼��ˮ������ӷ���ʽΪ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����
�ʴ�Ϊ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����
��4��̼������Һ�ڹ��̢�������������̼������ӷ���˫ˮ���������������Ͷ�����̼��̼������Һ�ڹ��̢��е���pH���ṩCO32-��ʹCo2+����ΪCoCO3��
�ʴ�Ϊ������pH���ṩCO32-��ʹCo2+����ΪCoCO3��
��5��A��Na2CO3��Һ�е���غ㣺c��Na+��+c��H+��=c��HCO3-��+c��OH-��+2c��CO32-������A����
B��̼������ӷ���ˮ���Լ�ˮ�ĵ��룬������Һ������Ũ�ȣ�c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+������B��ȷ��
C��̼������ӷ���ˮ���Լ�ˮ�ĵ��룬������Һ������Ũ�ȣ�c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+������C��ȷ��
D��Na2CO3��Һ�������غ㣺c��H+���Tc��OH-��+c��HCO3-��+2c��H2CO3������D��ȷ��
��ѡ��BCD��
��6��CoCl2?6H2O��CoCl2
238 130
m65mg
| 238 |
| m |
| 130 |
| 65mg |
A���ʵĻ�ѧʽΪCoCl2?nH2O�����У�
CoCl2?6H2O��CoCl2?nH2O��m
238 18��6-n��
119mg119mg-83mg
| 238 |
| 119mg |
| 18(6-n) |
| 119mg-83mg |
����A���ʵĻ�ѧʽΪ��CoCl2?2H2O���ʴ�Ϊ��CoCl2?2H2O��

��ϰ��ϵ�д�
 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
�����Ŀ