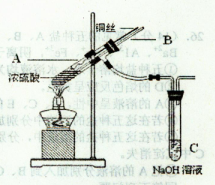
��Ŀ����
ijͬѧ��������ͭ����ᾧˮ�����IJⶨʵ�顣���������գ�
��ʵ�鲽�衿
��1����_______�����������ƣ���ͬ��ȷ������������������
��2���ڴ������м���Լ2 g��ϸ������ͭ���壬��������
��3����ʢ������ͭ����Ĵ������������������������ȣ�ֱ����ɫ��ȫ��ף�Ȼ�����������____________����ȴ�����£���������
��4���ظ�(3)��ʵ����к��ز�����ֱ�����γ������������0.001 g��
�����ݼ�¼�봦����
|
|
��һ��ʵ�� |
�ڶ���ʵ�� |
|
������������g�� |
29.563 |
30.064 |
|
������������������g�� |
31.676 |
32.051 |
|
���غ�����������ͭ��������g�� |
30.911 |
31.324 |
|
x��ֵ |
5.05 |
5.13 |
�����ϱ��е����ݴ�����������㱾��ʵ���������Ϊ______%����֪x������ֵΪ5����
�����������ۡ�
��1����һ��ʵ�飬������Ҫ����________�Σ������֣���ͬ����������Ҫ����_________�Ρ�
��2�����ز�����Ŀ����__________________��
��3���ظ�����ʵ����xƽ��ֵ��Ŀ����_____________________________��
��4��ʵ��ֵ������ֵƫ���ԭ�������________�����ţ���
a�����ȹ������о��彦�� b��������Ʒ�к��м��Ȳ��ӷ�������
c��ʵ��ǰ��������泱ʪ d���������պ�ֱ�ӷ��ڿ�������ȴ
������ƽ����������1.8%��2��4��ȷ������ʧȥ��ȫ���ᾧˮ����Сʵ������СżȻ��a��c��2�֣���
��������
�����������ʵ�鲽�衿��3���ڼ��Ⱥ���ȴʱ��Ϊ�˷�ֹ����ͭ��ˮ��Ӧ������ͭ���ڸ������н�����ȴ���Ӷ��õ��������������ˮ����ͭ��
�����ݼ�¼�봦��������������������������ȥʵ����������������������������
�����������ۡ�
��1������ͭ�ᾧˮ�����ⶨʵ����һ�������Ķ���ʵ�飬ʵ��Ĺؼ��Ǽ��ȹ�����ʹ�����еĽᾧˮȫ��ʧȥ��Ϊ�˱�֤ʧȥȫ���ᾧˮ��ʵ����Ҫ���ȡ��������ټ��ȡ��ٳ�����ֱ��������γ���ֵ������0.1g��0.1g��������ƽ�ĸ�������ע���ʵ������Ҫ����4�Σ����ƾ��塢�������;��塢����������ˮ����ͭ���ټ����ٳ��������أ�����һ�Σ�����ƣ���һĥ�������ijơ��������ȡ�����һ�㡱��
��2�����ز�����Ŀ����ȷ������ʧȥ��ȫ���ᾧˮ����Сʵ����
��3���ظ�����ʵ����xƽ��ֵ��Ŀ���Ǽ�СżȻ���
��4��a�����ȹ��������������彦��������ˮ�������ⶨ���ƫ��
b��������Ʒ�к��м��Ȳ��ӷ������ʣ��ᵼ�²ⶨ������ͭ������ƫ�ⶨ��ˮ������ƫС
c��ʵ��ǰ��������泱ʪ������ˮ�������ⶨ���ƫ��
d���������պ�ֱ�ӷ��ڿ�������ȴ�����岿����ˮ�����ֵ��С�����ƫС��
���㣺���鶨��ʵ���⡣

��Ϣʱ�������ǵ���������˼���ı�������ͬʱҲ�����˴����ĵ���������ij��ѧ��ȤС�齫һ����������·������õ�����Ҫ��Cu��Al������Fe��Au�Ƚ����Ļ���������������Ʊ�����ͭ����������������·�ߣ�����������������������ʽ����ʱ��Һ��pH���±�
| ������ | Fe��OH��3 | Al��OH��3 | Cu��OH��2 |
| ��ʼ���� | 1.1 | 4.0 | 5.4 |
| ��ȫ���� | 3.2 | 5.2 | 6.7 |
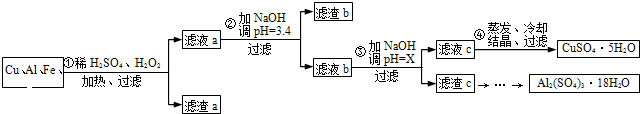
��1�����˲������õ��IJ���������______��
��2��Cu������ϡ������H2O2�Ļ����Һ�������ӷ���ʽ��______��
��3������a����Ҫ�ɷ���______��
��4���������X��ȡֵ��Χ��______��
��5�������Ƶõ�CuSO4?5H2O��ֻ��Na2SO4���ʣ����ø���Ʒ���С�����ͭ����ᾧˮ�����IJⶨ��ʵ�飬��ýᾧˮ����______���ƫ�͡�����ƫ�ߡ�����Ӱ�족������ͬѧȡ1.280g����Ʒ�����������سƵ�������Ϊ0.830g�������Ʒ�Ĵ���Ϊ______������С����ʾ��������λ��Ч���֣�