��Ŀ����
��8�֣���1����������� ���գ�
���գ�
��pH= a�� CH3COOH��Һϡ��100��������
CH3COOH��Һϡ��100�������� ��ҺpH a +2���>����<����
��ҺpH a +2���>����<����
��0.01mol/LCH3COOH��Һ��pH 2���>����<������
��2���۲�Ƚ���������С�⣬�Բ���֤��ij�ᣨHA����������ʵ�ԭ����������
һ�ǣ� ��
���ǣ� ��
��
��3�������������ԭ �����һЩ������֤��HA����������ʣ���ֻ�����д������������д�����岽�裬������Ŀ�ɲ�����Ҳ��������Ŀ��
�����һЩ������֤��HA����������ʣ���ֻ�����д������������д�����岽�裬������Ŀ�ɲ�����Ҳ��������Ŀ��
��
��
 ���գ�
���գ���pH= a��
 CH3COOH��Һϡ��100��������
CH3COOH��Һϡ��100�������� ��ҺpH a +2���>����<����
��ҺpH a +2���>����<������0.01mol/LCH3COOH��Һ��pH 2���>����<������
��2���۲�Ƚ���������С�⣬�Բ���֤��ij�ᣨHA����������ʵ�ԭ����������
һ�ǣ� ��
���ǣ�
 ��
����3�������������ԭ
 �����һЩ������֤��HA����������ʣ���ֻ�����д������������д�����岽�裬������Ŀ�ɲ�����Ҳ��������Ŀ��
�����һЩ������֤��HA����������ʣ���ֻ�����д������������д�����岽�裬������Ŀ�ɲ�����Ҳ��������Ŀ����
��
��8�֣�
��1����<����>��(2��)
��2��һ�ǣ���Һ�д��ڵ���ƽ�⣻(2��)
���ǣ�֤��HA������ȫ���롣(2��)
��3���ٶԱȵ����ʵ���Ũ�ȵ�HA��Һ�����ᵼ���ԶԱ�ʵ�飻
�ڲ�0.01mol/LHA��Һ��pH>2��
�۲�pH= a��HAϡ��100����������ҺpH<a +2
���к�10mLpH=1��HA��Һ����pH=13��NaOH��Һ���������10mL;
�����������������������𰸾��ɡ���2�֣�
��1����<����>��(2��)
��2��һ�ǣ���Һ�д��ڵ���ƽ�⣻(2��)
���ǣ�֤��HA������ȫ���롣(2��)
��3���ٶԱȵ����ʵ���Ũ�ȵ�HA��Һ�����ᵼ���ԶԱ�ʵ�飻
�ڲ�0.01mol/LHA��Һ��pH>2��
�۲�pH= a��HAϡ��100����������ҺpH<a +2
���к�10mLpH=1��HA��Һ����pH=13��NaOH��Һ���������10mL;
�����������������������𰸾��ɡ���2�֣�
��

��ϰ��ϵ�д�
�����Ŀ

 Ũ����ô�����H
Ũ����ô�����H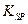 =2.6��10-39��Mg(OH)2���ܶȻ�����
=2.6��10-39��Mg(OH)2���ܶȻ�����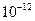 ��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��
��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��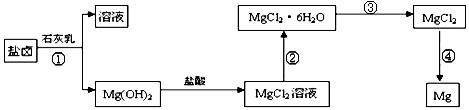
 .���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
.���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������