��Ŀ����
ijѧ����0.1000mol?L-1�������Һ�ⶨij�ռ���Ʒ�Ĵ��ȣ����ʲ������ᷴӦ����ʵ�鲽�����£�
��1�����ƴ���Һ����2.50g�����������ʵĹ����ռ���Ʒ����500mL��Һ�����õIJ������������ձ�����ͷ�ιܡ��������⣬����Ҫ
��2�����
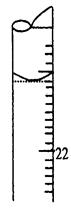
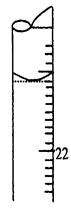
I��������ˮϴ����ʽ�ζ��ܣ�������ע���������Һ����0���̶�������
�̶��õζ��ܲ�ʹ�ζ��ܼ������Һ�壬����Һ������0����0���̶������£�����¼����
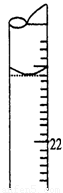
����ȡ20.00mL����Һע��ྻ����ƿ�У�������2�η�̪��Һ
�����ñ�Һ�ζ����յ㣬��¼�ζ���Һ�������
�������ζ��������д�����ǣ����ţ�
�ڲ�����еζ�ʱ�۾�Ӧ
��������������Ӷ�����������и��Ӷ��������ʹ�ⶨ���
��3������ȷ��������й����ݼ�¼���£�
���ռ���Ʒ�Ĵ���Ϊ
��1�����ƴ���Һ����2.50g�����������ʵĹ����ռ���Ʒ����500mL��Һ�����õIJ������������ձ�����ͷ�ιܡ��������⣬����Ҫ
500mL����ƿ����Ͳ
500mL����ƿ����Ͳ
����2�����
I��������ˮϴ����ʽ�ζ��ܣ�������ע���������Һ����0���̶�������
�̶��õζ��ܲ�ʹ�ζ��ܼ������Һ�壬����Һ������0����0���̶������£�����¼����
����ȡ20.00mL����Һע��ྻ����ƿ�У�������2�η�̪��Һ
�����ñ�Һ�ζ����յ㣬��¼�ζ���Һ�������
�������ζ��������д�����ǣ����ţ�
��
��
���ô�������ᵼ�²ⶨ���ƫ��
ƫ��
���ƫ����ƫС������Ӱ�족�����ڲ�����еζ�ʱ�۾�Ӧ
�۲���ƿ����Һ��ɫ�ı仯
�۲���ƿ����Һ��ɫ�ı仯
���жϵζ��յ����������Һ��ɫ��ȥ����30���ڲ���ɫ
��Һ��ɫ��ȥ����30���ڲ���ɫ
����������������Ӷ�����������и��Ӷ��������ʹ�ⶨ���
ƫ��
ƫ��
���ƫ����ƫС������Ӱ�족������3������ȷ��������й����ݼ�¼���£�
| �ζ����� | ����Һ��� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.20 | 20.38 |
| �ڶ��� | 20.00 | 4.00 | 24.20 |
| ������ | 20.00 | 2.38 | 22.60 |
80.8%
80.8%
%����������1����������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��ʹ�õ�������
��2����ʢ��Һ����ʽ�ζ��ܱ�����ϴ��������ȡ����ҺŨ��ƫ�ͣ�
�ڵζ�ʱ�۾�ע����ƿ����ɫ�仯�����ݴ���Һ�м����̪����ҺΪ��ɫ���кͷ�Ӧ��������Һ��ɫ��ʧ�����жϣ�
�۵ζ�ǰ���ӣ����¶���ƫ�ζ����ӻᵼ�¶���ƫС��
��3���ȷ������ݵ���Ч�ԣ�������������ƽ�������Ȼ����ݹ�ϵʽNaOH��HCl����������Ƶ����ʵ������ټ����ռ���Ʒ�Ĵ��ȣ�
��2����ʢ��Һ����ʽ�ζ��ܱ�����ϴ��������ȡ����ҺŨ��ƫ�ͣ�
�ڵζ�ʱ�۾�ע����ƿ����ɫ�仯�����ݴ���Һ�м����̪����ҺΪ��ɫ���кͷ�Ӧ��������Һ��ɫ��ʧ�����жϣ�
�۵ζ�ǰ���ӣ����¶���ƫ�ζ����ӻᵼ�¶���ƫС��
��3���ȷ������ݵ���Ч�ԣ�������������ƽ�������Ȼ����ݹ�ϵʽNaOH��HCl����������Ƶ����ʵ������ټ����ռ���Ʒ�Ĵ��ȣ�
����⣺��1����2.50g�����������ʵĹ����ռ���Ʒ����500mL��Һ�����ƹ���Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ�ҡ�ȵȣ���Ҫ��������������ƽ���ձ�������������Ͳ��500mL����ƿ����ͷ�ιܣ����õIJ������������ձ�����ͷ�ιܡ��������⣬����Ҫ��Ͳ��500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ����Ͳ��
��2����������ˮϴ����ʽ�ζ��ܣ�Ȼ��������ʽ�ζ����ñ�Һ������ϴ������ᵼ�±�ҺŨ�ȼ�С���õ�ʱ���ĵı�Һ������ⶨ���ƫ��
�ʴ�Ϊ���� ƫ��
�ڵζ�ʱ˫��Ӧע��۲���ƿ����Һ��ɫ�ı仯����ƿ�е����̪����ҺΪ��ɫ�����ŷ�Ӧ���У�����������������ǡ�÷�Ӧ����Һ����ɫ����ʧ�����Դﵽ�յ�����Ϊ����Һ��ɫ��ȥ����30���ڲ���ɫ��
�ʴ�Ϊ���۲���ƿ����Һ��ɫ�ı仯����Һ��ɫ��ȥ����30���ڲ���ɫ��
�۲���II�����Ӷ������ᵼ�µζ��ܵĶ���ƫ������и��Ӷ������ᵼ�µζ��ܶ���ƫС�����յ������ĵ��������ƫС���ⶨ���ƫ�ͣ�
�ʴ�Ϊ��ƫС��
��3������������������ֱ�Ϊ��20.38mL-0.20mL=20.18mL��24.20mL-4.00mL=20.20mL��22.6mL-2.38mL=20.22mL�����εζ����ݶ�����Ч�ģ��������������ƽ�����Ϊ��
=20.20mL��
���ݹ�ϵʽNaOH��HCl��֪��n��NaOH��=n��HCl��=0.1000mol?L-1��0.022mL=0.00202mol��
����20.00mL������Һ���У�m���ռ�Tn?M�T0.00202mol��40g/mol=0.808g��
����1000mL������Һ����m���ռ�T0.808g��
=2.02g��
�ռ�Ĵ��Ȧأ��ռ=
��100%=80.8%��
�ʴ�Ϊ��80.8%��
�ʴ�Ϊ��500mL����ƿ����Ͳ��
��2����������ˮϴ����ʽ�ζ��ܣ�Ȼ��������ʽ�ζ����ñ�Һ������ϴ������ᵼ�±�ҺŨ�ȼ�С���õ�ʱ���ĵı�Һ������ⶨ���ƫ��
�ʴ�Ϊ���� ƫ��
�ڵζ�ʱ˫��Ӧע��۲���ƿ����Һ��ɫ�ı仯����ƿ�е����̪����ҺΪ��ɫ�����ŷ�Ӧ���У�����������������ǡ�÷�Ӧ����Һ����ɫ����ʧ�����Դﵽ�յ�����Ϊ����Һ��ɫ��ȥ����30���ڲ���ɫ��
�ʴ�Ϊ���۲���ƿ����Һ��ɫ�ı仯����Һ��ɫ��ȥ����30���ڲ���ɫ��
�۲���II�����Ӷ������ᵼ�µζ��ܵĶ���ƫ������и��Ӷ������ᵼ�µζ��ܶ���ƫС�����յ������ĵ��������ƫС���ⶨ���ƫ�ͣ�
�ʴ�Ϊ��ƫС��
��3������������������ֱ�Ϊ��20.38mL-0.20mL=20.18mL��24.20mL-4.00mL=20.20mL��22.6mL-2.38mL=20.22mL�����εζ����ݶ�����Ч�ģ��������������ƽ�����Ϊ��
| 20.18mL+20.20mL+20.22mL |
| 3 |
���ݹ�ϵʽNaOH��HCl��֪��n��NaOH��=n��HCl��=0.1000mol?L-1��0.022mL=0.00202mol��
����20.00mL������Һ���У�m���ռ�Tn?M�T0.00202mol��40g/mol=0.808g��
����1000mL������Һ����m���ռ�T0.808g��
| 500mL |
| 20mL |
�ռ�Ĵ��Ȧأ��ռ=
| 2.02g |
| 2.5g |
�ʴ�Ϊ��80.8%��
���������⿼���к͵ζ�������һ�����ʵ���Ũ�ȵ���Һ����ѡ��ע��������к͵ζ�ʱ�����ֿ��ƻ�������С������ҡ����ƿ���۾�ע����ƿ����ɫ�仯�������Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ