��Ŀ����
�������ط�ұ��þ�Ĺ�����������ͼ��¯���г������հ���ʯ�����⣬���������չ���������Al2O3����ҪĿ���ǽ��������۵㣬����Һ̬������
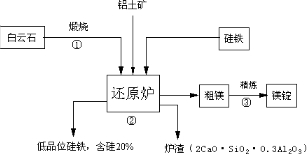
��֪����ʯ�ijɷ�ΪCaCO3��MgCO3,������ָ����55%�����ҵ���[�ɱ�ʾΪSi(Fe)]������ԭ��,���õ���Ʒλ�Ĺ���[�ɱ�ʾΪFe(Si)]����������ͼ����
�Իش���������
��1����Ӧ�٣�����ʯ���յ�CaO��MgO�Ļ�ѧ����ʽΪ�� ��
��2����Ӧ�ڣ���ԭ¯�з�����Ӧ����þ��¯���Ļ�ѧ����ʽΪ�� ��
��3���óɷֵĻ�����һ�ֹ�ҵ��������Ҫԭ�ϣ�����Ϊ���� ԭ�ϡ�
��4���������ط�ұ��þ��һ̨4500ǧ�ߵ�¯�ӿ��ղ�Լ7.2��þ��һ������Լ���ĺ���60%�Ĺ��� �֡�
��1��CaCO3��MgCO3![]() CaO��MgO+2CO2��
CaO��MgO+2CO2��
��2��2(CaO��MgO)��Si(Fe)��0.3Al2O3 ![]() 2Mg��2CaO��SiO2��0.3Al2O3��Fe(Si)
2Mg��2CaO��SiO2��0.3Al2O3��Fe(Si)
[��2(CaO��MgO)��Si��0.3Al2O3 ![]() 2Mg��2CaO��SiO2��0.3Al2O3]
2Mg��2CaO��SiO2��0.3Al2O3]
��3��ˮ�� ��4��8.4t
����:
���⿼�鹤��������þ��ұ������2�������������������е���Ϣ����ԭ¯��Ϊ����ʯ���ղ������������������������ԭ��Ӧ������¯����þ�Լ���Ʒλ�Ĺ���������ʽΪ��2(CaO��MgO)��Si(Fe)��0.3Al2O3 ![]() 2Mg��2CaO��SiO2��0.3Al2O3��Fe(Si)����3����¯����Ҫ�ɷ�Ϊ�����Σ�������������ˮ��ȹ����β�Ʒ����4���ɹ�ϵ��
2Mg��2CaO��SiO2��0.3Al2O3��Fe(Si)����3����¯����Ҫ�ɷ�Ϊ�����Σ�������������ˮ��ȹ����β�Ʒ����4���ɹ�ϵ��
2Mg��Si
48 28
7.2 x
X=4.2t
�������Ĺ�Ϊ4.2t��
��ԭ�й�0.6x�������Ʒ�������й�0.6x-4.2
��Ӧ��Ʒλ��������ˣ�x-4.2���ɵã� =0.2��x=8.4t��