��Ŀ����
���л�ѧʵ�飬�۲�ʵ������ͨ�����������ó���ȷ�Ľ�����ѧϰ��ѧ�ķ���֮һ��������ʵ����ʵ�Ľ�����ȷ����
| A����KI������Һ��ͨ��Cl2����Һ������˵������������۷�����ɫ��Ӧ |
| B��Ũ�����ڹ��������±��,˵��Ũ����ȶ�,����ɫ����������������Ũ���� |
| C����ij��Һ�м���HNO3�ữ��BaCl2��Һ�а�ɫ��������,˵����Һ�к���SO42- |
| D����ͭƬ����Ũ������������ʵ������˵��ͭ�����Ũ�����з����ۻ� |
B
A ����Cl2��2KI=2KCl��I2 �������۷�����ɫ��Ӧ��
B ��ȷ���ڹ����£�4HNO3=4NO2��O2��2H2O NO2Ϊ����ɫ������Ũ�����Ի�ɫ
C ������Һ�п��ܺ���SO42����Ҳ���ܺ���SO32��
D ����ͭ�����Ũ�����в�û���������ܵ�����Ĥ��û�з����ۻ����ڼ���ʱ�ŷ�Ӧ��
B ��ȷ���ڹ����£�4HNO3=4NO2��O2��2H2O NO2Ϊ����ɫ������Ũ�����Ի�ɫ
C ������Һ�п��ܺ���SO42����Ҳ���ܺ���SO32��
D ����ͭ�����Ũ�����в�û���������ܵ�����Ĥ��û�з����ۻ����ڼ���ʱ�ŷ�Ӧ��

��ϰ��ϵ�д�
�����Ŀ
 ��V2O5(����)��ͨ��SO2ʱ����Kͨ������O2�Ļ�ѧ��Ӧ����ʽΪ ��
��V2O5(����)��ͨ��SO2ʱ����Kͨ������O2�Ļ�ѧ��Ӧ����ʽΪ �� �� C���������
�� C��������� Һ D��������Һ
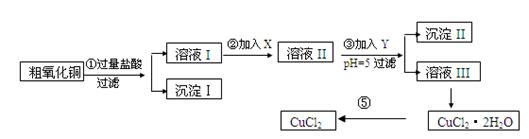
Һ D��������Һ l2�뺬X����Һ��Ӧ�����ӷ���ʽ ��
l2�뺬X����Һ��Ӧ�����ӷ���ʽ ��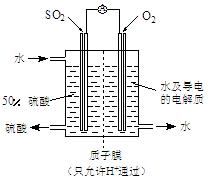

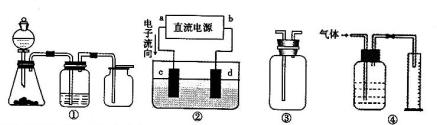
 ��aΪ���⣬dΪ����
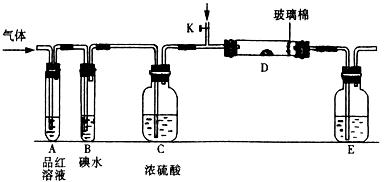
��aΪ���⣬dΪ���� 2��NH3��Cl2,��HCl��NO2��
2��NH3��Cl2,��HCl��NO2�� ��װ�â������ڲ����������
��װ�â������ڲ����������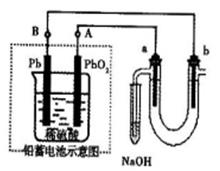
 ���ԭϡ��Һ��I-Ũ�ȣ��ζ�ʱ�ķ�ӦΪ��2Na2S2O3 + I2 = Na2S4O6 + 2NaI���Իش�
���ԭϡ��Һ��I-Ũ�ȣ��ζ�ʱ�ķ�ӦΪ��2Na2S2O3 + I2 = Na2S4O6 + 2NaI���Իش� ____________����ǡ����ǡ�������ΪAgX�����������Ļ�ѧʽΪ__________������ΪAgX��������˿ղ����
____________����ǡ����ǡ�������ΪAgX�����������Ļ�ѧʽΪ__________������ΪAgX��������˿ղ����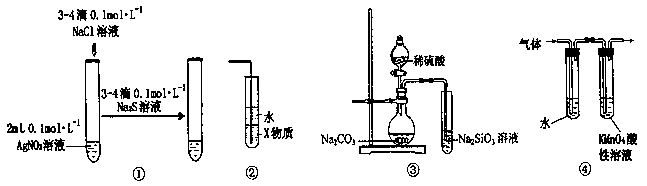
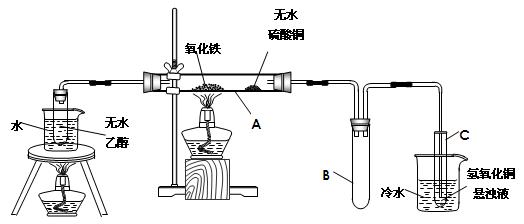

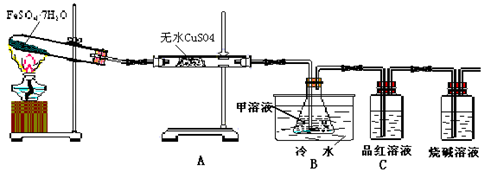
 Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺
Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺