��Ŀ����
����������ȷ����
A��������pH=7��CH3COOH��CH3COONa�Ļ��Һ�����ӵ�Ũ�ȴ�С˳��Ϊ��c��Na+��> c��CHCOO-��> c��H+��= c��OH-��
B��0.1 mol/L KHS����Һ�У�c��K+��= 2c��S2-��+ c��HS-��+ c��H2S��
C��25�� ʱ��pH��Ϊ12��NaOH��Һ��Na2CO3��Һ����ˮ�������c��OH-����ǰ��С�ں���
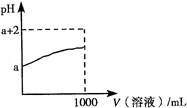
D���к�pH�������ͬ�������������Һ������NaOH�����ʵ������
���𰸡�
C
��������
������������ݵ���غ��֪��c��Na+����c��H+����c��OH-����c��CHCOO-����Һ�����ԣ���c��Na+���� c��CHCOO-��> c��H+��= c��OH-����A����ȷ��B����ȷ�������������غ㣬Ӧ����c��K+����c��S2-��+ c��HS-��+ c��H2S��������������ǿ�����ˮ�ĵ��룬̼����ˮ��ٽ�ˮ�ĵ��룬����C��ȷ�����������ᣬ��pH��ͬ�������£������Ũ�ȴ�������ģ���˴������ĵ��������ƶ࣬D����ȷ����ѡC��
���㣺������Һ������Ũ�ȴ�С�Ƚ�
���������ж���Һ������Ũ�ȴ�Сʱ��һ��Ҫ������úü����غ��ϵ��������غ㡢�����غ�������غ㡣

��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ
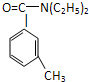 ���ð�������DEET����һ�ֶ��˰�ȫ�����Ը�����ҩ�Ե��������ü�����ṹ��ʽΪ����֪��RCOOH
���ð�������DEET����һ�ֶ��˰�ȫ�����Ը�����ҩ�Ե��������ü�����ṹ��ʽΪ����֪��RCOOH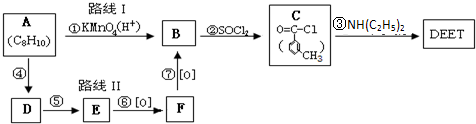
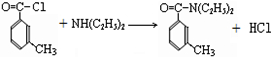
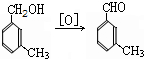
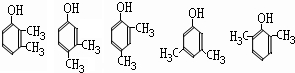 ����д2�֣�
����д2�֣�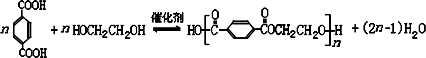
 NH3?H2O+H+
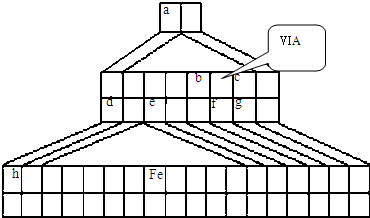
NH3?H2O+H+ ��2010?��������ģ��X��Y��Z��W��Ϊ����10���ӵ���������X��Y��ZΪ���ӣ�WΪ���ӣ���X��Z�����к��еĹ��õ��Ӷ���֮��Ϊ3��4��
��2010?��������ģ��X��Y��Z��W��Ϊ����10���ӵ���������X��Y��ZΪ���ӣ�WΪ���ӣ���X��Z�����к��еĹ��õ��Ӷ���֮��Ϊ3��4��