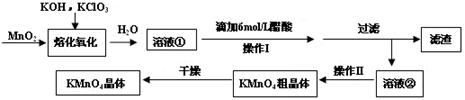
��Ŀ����
�������ƣ�NaH2PO2�������ڻ�ѧ������
��1����ѧ��������Һ�к���Ni2+��H2PO2���������������·������¶�����Ӧ��
�� Ni2+ + H2PO2��+ �� Ni + H2PO3��+
�� 6H2PO2- +2H+ ��2P+4H2PO3-+3H2��
����ƽ��Ӧʽ�١�
��2����Ӧʽ���л�ԭ���� ������ԭԪ���� ��
��3����Ӧ���У�������1 mol H2PO3-����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ mol��
��4���Ӷ�����Ӧ������������1mol Ni��1mol P��ͬʱ�� mol H2PO3�����ɡ�
��5��������и�С���еĻ�ѧ��Ӧ����ʽ��
�������O2��Ӧ����������ϸ��ӣ�����ͨ�������K2O�����������K2O2�����г�������ͳ�������ȡ�Ҫ�Ʊ����������ͨ������һ�����ü������ԭ��Ӧ�Ĺ�����������λ��������Ρ�д�����л�ѧ��Ӧʽ��
������������Ʒ�Ӧ
�ڼػ�ԭ����أ�ͬʱ��������һ�ֵ�������
��1��Ni2++H2PO2+H2O=Ni+H2PO3+2H+ ��2�� H2PO2 Ni ��3��2mol ��4��3 ��5����2Na+Na2O2= 2Na2O
��10K+2KNO3=6K2O+N2
��������������۲췴Ӧʽ1��֪����+2�۱��0�ۣ����ϼ۽���2��H2PO2���е�PΪ+1�۱�ΪH2PO3���е�P+3�ۣ����ϼ�����2���ݵ�ʧ����������ȵ�ԭ��������H2PO2������Ҫ�����κε������پ�ԭ�Ӹ����غ��֪����߷�Ӧ��ֻ�������������ұ�������������ڷ�Ӧ���п϶�����һ����ԭ�ӣ�����Ŀ��˵���÷�Ӧʱ�����������½��У����Է�Ӧ����Ӧ����ˮ�����˷�Ӧ�����Կ��Եó�Ni2++H2PO2+H2O=Ni+H2PO3+2H+
H2PO2�� ��P���ϼ����ߣ��������ԭ������Ni2+���ϼ۽����������������Ա���ԭ��Ԫ������
6H2PO2- +2H+ ��2P+4H2PO3-+3H2�� 6H2PO2-��6��P�Ļ��ϼ���+1���2��0�۵�P��4��+3�۵�4H2PO3�е�P���������4Ħ����H2PO3-����ת����4����2��������1Ħ����H2PO3-����ת�Ƶ����ʵ���Ϊ2Ħ����
Ni2++H2PO2+H2O=Ni+H2PO3+2H+ ��֪����һĦ����Ni��ͬʱ����1Ħ����H2PO3��6H2PO2- +2H+ ��2P+4H2PO3-+3H2����֪����1Ħ����P��ͬʱ����2Ħ����H2PO3�������ܹ�����3Ħ��H2PO3��
���Ʊ����������ͨ������һ�����ü������ԭ��Ӧ�Ĺ�����������λ��������Ρ���֪
��2Na+Na2O2= 2Na2O ��10K+2KNO3=6K2O+N2
���㣺���黯ѧʽ����ƽ��������ԭ��Ӧ�����ʵ�������ؼ����֪ʶ

��֪NO2��ˮ������Ӧ��2NO2 �� H2O �� HNO2 �� HNO3��ʵ��֤����NO2ͨ��ˮ�л��ݳ�NO���壬��ͨ��NaOH��Һ�������ȫ�����գ�ͨ��Na2CO3��Һ��ֻ�ݳ�CO2������˵���������
| A��HNO2�dz����ȶ�����ֽ����ΪNO��H2O |
| B��NO2��ˮ�ķ�Ӧ������NO2����ˮ����᪻���Ӧ����HNO2��HNO3��HNO2�ٷֽ� |
| C��HNO2����������̼������� |
| D��NO2ͨ��NaOH��Һ�У����ɵ�HNO2��HNO3����NaOH�����кͷ�Ӧ |
���к�Fe(NO3)3��Cu(NO3)2��HNO3��ijϡ��Һ�����������������ۣ���Һ��Fe2+Ũ����������۵����ʵ���֮��Ĺ�ϵ��ͼ��ʾ�������Һ��Fe(NO3)3��Cu(NO3)2��HNO3���ʵ���Ũ��֮��Ϊ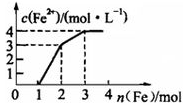
| A��1��1��3 | B��1��2��4 | C��1��1��4 | D��1��3��1 |
�ж������йػ�ѧ���������������ȷ����
| A�����壺������ʵ�ֱ���Ƿ���1nm��100nm֮�� |
| B��������ԭ��Ӧ����Ӧǰ��Ԫ�صĻ��ϼ��Ƿ����˱仯 |
| C�����ۻ������ɻ������Ԫ���Ƿ�ȫ�����Ƿǽ���Ԫ�� |
| D����ѧ�仯���Ƿ�����ЧӦ����ɫ�仯�������������ɵ�����ʵ������ |
(14��)���ռ�������ˮ�����ξ��ơ���һ�ξ�����Ҫ���ó�������ȥ����ˮ��Ca2����Mg2����SO42�������ӣ��������£�
��. �����ˮ�м������BaCl2��Һ�����ˣ�
��. ��������Һ�м������Na2CO3��Һ�����ˣ�
��. ��Һ���������pH����õ�һ�ξ�����ˮ��
��1�����̢��ȥ��������______��
��2�����̢����ɵIJ��ֳ��������ܽ��(20 ��/g)���±��������ݱ������ݽ����������⣺
| CaSO4 | Mg2(OH)2CO3 | CaCO3 | BaSO4 | BaCO3 |
| 2.6��10��2 | 2.5��10��4 | 7.8��10��4 | 2.4��10��4 | 1.7��10��3 |
�ٹ��̢�ѡ��BaCl2����ѡ��CaCl2��ԭ��Ϊ___________________________________��
�ڹ���II֮����Ca2����Mg2��������Ba2���Ƿ����ʱ��ֻ����Ba2�����ɣ�ԭ����____________��
��3���ڶ��ξ���Ҫ��ȥ����I����IO3�� ��NH4�� ��Ca2����Mg2��������ʾ�����£�
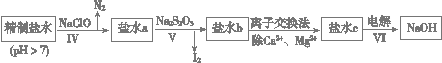
�� ���̢���ȥ��������______��_______��
�� ��ˮb�к���SO42����Na2S2O3��IO3�� ��ԭΪI2�����ӷ���ʽ��___________________________��
�� ����VI�У���ƷNaOH�ڵ��۵�__________������(���������������)���õ���Ϊ______���ӽ���Ĥ����(�����������)��
���������Ǵ�����Ⱦ��֮һ��������������ķ����ж��֡�
��1�����ü������ԭ���������֪��
��CH4 (g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g�� ��H ����574 kJ/mol
��CH4(g)��4NO(g���� 2N2(g)��CO2(g)��2H2O(g�� ��H ����1160 kJ/mol
��CH4 ��NO2 ��ԭΪN2 ���Ȼ�ѧ����ʽΪ�� ��
��2������NH3����ԭ�������SCR����)���ü�����ĿǰӦ����㷺���������������ѳ������� ��Ӧ�Ļ�ѧ����ʽΪ: Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1�����ɣ���
Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1�����ɣ���
��3������ClO2�������������ת����������: NO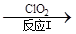 NO2
NO2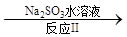 N2����֪��Ӧ��Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O ��NO2 + HNO3 + HCl����Ӧ��Ļ�ѧ����ʽ�� ��������11.2 L N2����״������������ClO2 g ��
N2����֪��Ӧ��Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O ��NO2 + HNO3 + HCl����Ӧ��Ļ�ѧ����ʽ�� ��������11.2 L N2����״������������ClO2 g ��
��4���û���̿��ԭ��������������йط�ӦΪ��C��s��+2NO��g�� N2 ��g��+CO2 ��g����H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2 ��g��+CO2 ��g����H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
| Ũ��/mol?L-1/ ʱ��/min | NO | N2 | CO2 |
| 0 | 0.100 | 0 | 0 |
| 10 | 0.058 | 0.021 | 0.021 |
| 20 | 0.040 | 0.030 | 0.030 |
| 30 | 0.040 | 0.030 | 0.030 |
| 40 | 0.032 | 0.034 | 0.017 |
| 50 | 0.032 | 0.034 | 0.017 |
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K= ��������λС��������30min�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������ ������30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3����÷�Ӧ�ġ�H 0�����������=����������