��Ŀ����
|
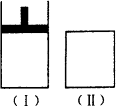
��ͼ����һ���¶��£���Ϊ��ѹ�ܱ���������Ϊ�����ܱ��������ڢ��зֱ����2 mol��A��2 mol��B����ʼʱ���������ΪVL���������·�Ӧ���ﵽ��ѧƽ��״̬��2AʮB
| |
A�� |
x��ֵΪ2 |
B�� |
B���ʿ�Ϊ�����Һ�� |
C�� |
�������д���ʼ��ƽ������ʱ����ͬ |
D�� |
ƽ��ʱ�������������С��V L |
�𰸣�C

��ϰ��ϵ�д�
�����Ŀ
 ��2009?��Զģ�⣩�¹��˹�����1909�귢���ĺϳɰ���Ӧԭ��Ϊ��N2��g��+3H2��g��?2NH3��g�� ��֪298Kʱ����H=-92.4kJ?mol-1�Իش��������⣺
��2009?��Զģ�⣩�¹��˹�����1909�귢���ĺϳɰ���Ӧԭ��Ϊ��N2��g��+3H2��g��?2NH3��g�� ��֪298Kʱ����H=-92.4kJ?mol-1�Իش��������⣺