��Ŀ����
��8�֣�A��B��C��D��EΪ������Ԫ�أ�A-Eԭ��������������������֮��Ϊ40��B��Cͬ���ڣ�A��Dͬ���壬A��C���γ�����Һ̬������A2C��A2C2��E�ǵؿ��к������Ľ���Ԫ�ء�
��1��BԪ�������ڱ��е�λ��Ϊ___________________________��
��2����D�ĵ���Ͷ��A2C�У���Ӧ��õ�һ����ɫ��Һ��E�ĵ����ڸ���ɫ��Һ�з�Ӧ�����ӷ���ʽΪ___________________________________________��
(3)Ԫ��D�ĵ�����һ�������£�����A���ʻ��������⻯��DA���۵�Ϊ800��C��DA����ˮ��Ӧ�ų���������ѧ��Ӧ����ʽΪ____________________________
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2C2��ϡ������ݼȴﵽ������Ŀ�ģ��ֱ����˻�������д����Ӧ�Ļ�ѧ����ʽ____________________________________________��
��1��BԪ�������ڱ��е�λ��Ϊ___________________________��
��2����D�ĵ���Ͷ��A2C�У���Ӧ��õ�һ����ɫ��Һ��E�ĵ����ڸ���ɫ��Һ�з�Ӧ�����ӷ���ʽΪ___________________________________________��
(3)Ԫ��D�ĵ�����һ�������£�����A���ʻ��������⻯��DA���۵�Ϊ800��C��DA����ˮ��Ӧ�ų���������ѧ��Ӧ����ʽΪ____________________________
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2C2��ϡ������ݼȴﵽ������Ŀ�ģ��ֱ����˻�������д����Ӧ�Ļ�ѧ����ʽ____________________________________________��
��1���ڶ����ڢ�A�� ��2��2Al + 2H2O + 2OH- = 2AlO2- + 3H2��
(3) NaH + H2O = H2�� + NaOH
(4)H2O2 + H2SO4 + Cu = CuSO4 + 2H2O ��ÿ��2�֣���8�֣�
(3) NaH + H2O = H2�� + NaOH
(4)H2O2 + H2SO4 + Cu = CuSO4 + 2H2O ��ÿ��2�֣���8�֣�
E�ǵؿ��к������Ľ���Ԫ�أ���E������A��C���γ�����Һ̬������A2C��A2C2������A��H��C��O��A��Dͬ���壬����D���ơ�������֮��Ϊ40������B��N��
��1����Ԫ�ص�ԭ��������7��λ�ڵڶ����ڢ�A��
��2���ƺ�ˮ��Ӧ�����������ƣ������������Ʒ�Ӧ����������ƫ�����ƣ�����ʽΪ2Al + 2H2O + 2OH- = 2AlO2- + 3H2����
��3��H��Na�γ����ӻ������⻯�ƣ���ˮ��Ӧ�����������ƺ�����������ʽΪ NaH + H2O = H2�� + NaOH��
��4��˫��ˮ���������ԣ��һ�ԭ������ˮ��û����Ⱦ������ʽΪH2O2 + H2SO4 + Cu = CuSO4 + 2H2O��
��1����Ԫ�ص�ԭ��������7��λ�ڵڶ����ڢ�A��
��2���ƺ�ˮ��Ӧ�����������ƣ������������Ʒ�Ӧ����������ƫ�����ƣ�����ʽΪ2Al + 2H2O + 2OH- = 2AlO2- + 3H2����
��3��H��Na�γ����ӻ������⻯�ƣ���ˮ��Ӧ�����������ƺ�����������ʽΪ NaH + H2O = H2�� + NaOH��
��4��˫��ˮ���������ԣ��һ�ԭ������ˮ��û����Ⱦ������ʽΪH2O2 + H2SO4 + Cu = CuSO4 + 2H2O��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
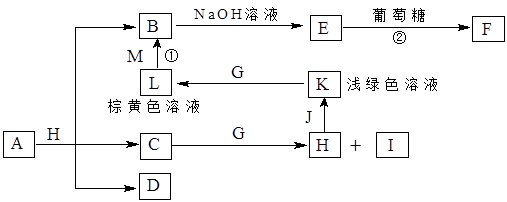

 A+L�Ļ�ѧ����ʽΪ�� ��
A+L�Ļ�ѧ����ʽΪ�� �� ��
�� ��
�� ����Ч�����Ƹΰ�����ͬλ��ԭ�Ӻ��ڵ�����������������֮����
����Ч�����Ƹΰ�����ͬλ��ԭ�Ӻ��ڵ�����������������֮����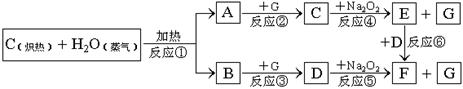
 ��E
��E  ����NA��ʾ����ӵ�������
����NA��ʾ����ӵ������� ol��
ol��