��Ŀ����
��֪ij��Һ��ֻ����OH�D��H+��Na+��CH3COO�D�������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С
˳�����������ֹ�ϵ��
�٣�CH3COO�D����c��Na+����c��H+����c��OH�D��
��c��Na+����c��OH�D����c��CH3COO�D����c��H+��
��c��Na+����c��CH3COO�D����c��OH�D����c��H+��
��c��CH3COO�D����c��H+����c��Na+��c��OH�D��
��д���пհף�
��1������Һ��ֻ�ܽ���һ�����ʣ���������� ��������������Ũ�ȵĴ�С˳
��Ϊ ������ţ�
��2����������ϵ�Т�����ȷ�ģ�����Һ�е�����Ϊ ��
��������ϵ�Т�����ȷ�ģ�����Һ�е�����Ϊ ��
��3��������Һ���������ȵĴ��������������Һ��϶��ɣ���ǡ�ó����ԣ�����ǰ
c��NaOH�� c��CH3COO��������ڡ�����С�ڡ����ڡ�����ͬ����
���ǰ����c��H+���ͼ���c��OH�D���Ĺ�ϵ��c��H+�� c��OH�D��
��1��NaAC��1�֣� �ۣ�2�֣�
��2��NaOH NaAC��2�֣� HAC NaAC��2�֣�
��3��С�ڣ�2�֣� С�ڣ�1�֣�

 С�����ϵ�д�
С�����ϵ�д�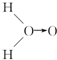 ��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѣ�
��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѣ�
 �ķе����
�ķе����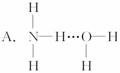

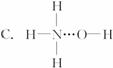

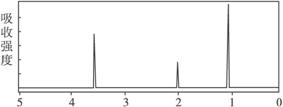
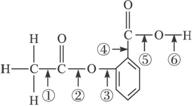
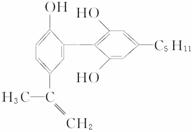


 �ӷ�Ӧʽ�� ��
�ӷ�Ӧʽ�� ��