��Ŀ����
ijѧϰС����ͨ����ӦNa2S2O3+H2SO4�TNa2SO4+S��+SO2��+H2O�о���Ӧ���ʵ�Ӱ�����غ�Na2S2O3��������Ȥ��������֪Na2S2O3����Ϊ��������ƣ��׳ƺ��������Կ�������һ��Sԭ��ȡ����Na2SO4�е�һ��Oԭ�Ӷ��γɣ���ʵ��С���������ѧϰ��˼��Ԥ����Na2S2O3��ijЩ���ʣ���ͨ��ʵ��̽����֤�Լ���Ԥ�⣮��������衿
��1������ѧ����ΪNa2S2O3��N2SO4�ṹ���ƣ���ѧ����Ҳ���ƣ��������ʱNa2S2O3��Һ��pH
��2������ѧ����SԪ�ػ��ϼ��Ʋ�Na2S2O3��SO2�������ƣ������н�ǿ��
��ʵ��̽����
ȡ����Na2S2O3���壬����ˮ���Ƴ�Na2S2O3��Һ����������̽������д���пո�
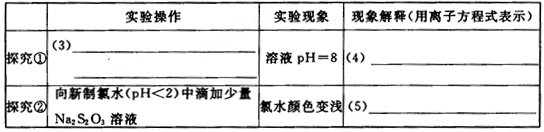
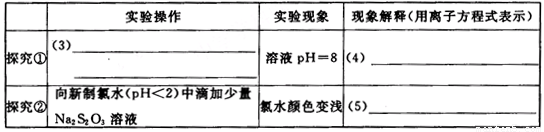
| ʵ����� | ʵ������ | ������ͣ������ӷ���ʽ��ʾ�� | |
| ̽���� | ��3�� |
��ҺpH=8 | ��4�� |
| ̽���� | ��������ˮ��pH��2���еμ�����Na2S2O3��Һ | ��ˮ��ɫ��dz | ��5�� |
��6��̽���٣�
��7��̽���ڣ�
���������ۡ�
��8����ͬѧ��̽���ڡ���Ӧ�����Һ�еμ���������Һ���۲쵽�а�ɫ�������������ݴ���Ϊ��ˮ�ɽ�Na2S2O3����������Ϊ�÷����Ƿ���ȷ��˵������
��9������������ƶ���ʵ�鷽����֤��Na2S2O3����ˮ��������ķ�����
��������1������Na2S2O3��Na2SO4�ṹ���ƣ���ѧ����Ҳ���ƻش��жϣ�
��2������Na2S2O3��SO2�������ƣ�����������н�ǿ�Ļ�ԭ���жϣ�
��3��������Һ����ԣ���PH��ֽ���вⶨ������pH�IJⶨ��������жϣ�
��4��������Һ�Լ��ԣ�˵��������������ˮ�����ˮ�ⷽ��ʽ����д�������ش�
��5����ˮ����������Ʒ�Ӧ����ˮ��dz˵������������Ӧ�����������������������Ϊ�����ƣ�
��6���������ʺ�����˵���������������Һ��ˮ���Լ��ԣ�
��7���������ʺ�����˵����������ƾ��л�ԭ�ԣ�
��8����ˮ������Һ�к��������ӣ�
��9��������Һ���Ƿ�����������Ӵ�����˵����������Ʊ�����������
��2������Na2S2O3��SO2�������ƣ�����������н�ǿ�Ļ�ԭ���жϣ�
��3��������Һ����ԣ���PH��ֽ���вⶨ������pH�IJⶨ��������жϣ�
��4��������Һ�Լ��ԣ�˵��������������ˮ�����ˮ�ⷽ��ʽ����д�������ش�
��5����ˮ����������Ʒ�Ӧ����ˮ��dz˵������������Ӧ�����������������������Ϊ�����ƣ�
��6���������ʺ�����˵���������������Һ��ˮ���Լ��ԣ�
��7���������ʺ�����˵����������ƾ��л�ԭ�ԣ�
��8����ˮ������Һ�к��������ӣ�
��9��������Һ���Ƿ�����������Ӵ�����˵����������Ʊ�����������
����⣺��1������Na2S2O3��Na2SO4�ṹ���ƣ���ѧ����Ҳ���ƣ���������ǿ��ǿ���Σ�����pH=7����֪���������Ҳ��pH=7��
�ʴ�Ϊ��=��
��2������������н�ǿ�Ļ�ԭ�ԣ�Na2S2O3��SO2�������ƣ�������������ƾ��н�ǿ�Ļ�ԭ�ԣ�
�ʴ�Ϊ����ԭ�ԣ�
��3���������ʵ��̽���ٵ�Ŀ���Dzⶨ��Һ��pH������Ϊ�ò�����պȡNa2S2O3��Һ����pH��ֽ�в�������ֽ��ɫ�����ɫ�����գ�
�ʴ�Ϊ���ò�����պȡNa2S2O3��Һ����pH��ֽ�в�������ֽ��ɫ�����ɫ�����գ�
��4���ⶨ��ҺpH=8˵����Һ�ʼ��ԣ�֤��������������ˮ���Լ��ԣ���Ӧ�����ӷ���ʽΪ��S2O32-+H2O?HS2O3-+OH-��
�ʴ�Ϊ��S2O32-+H2O?HS2O3-+OH-��
��5����������ˮ��pH��2���еμ�����Na2S2O3��Һ����ˮ����������Ʒ�Ӧ��ˮ��ɫ��dz˵������������Ӧ�����������������������Ϊ�����ƣ���Ӧ�����ӷ���ʽΪ��S2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+��
�ʴ�Ϊ��S2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+��
��6������̽�����������������ҺpH=8��֤��Na2S2O3���м��ԣ�
�ʴ�Ϊ��Na2S2O3���м��ԣ�
��7����������ˮ��pH��2���еμ�����Na2S2O3��Һ����ˮ����������Ʒ�Ӧ��ˮ��ɫ��dz˵������������Ӧ�����������������������Ϊ�����ƣ�֤��Na2S2O3���л�ԭ�ԣ�
�ʴ�Ϊ��Na2S2O3���л�ԭ�ԣ�
��8��̽��������������ˮ��pH��2���еμ�����Na2S2O3��Һ����ˮ������Һ��һ���������ӣ��������������ɳ�������֤�������������������ɵģ����Է�������ȷ��
�ʴ�Ϊ������ȷ������ˮ��������ˮ��ͬ������Cl-��
��9��֤��Na2S2O3����ˮ����������������������Ʊ������������ɵ���������Ӽ�С���飬����Ϊȡ������Ӧ�����Һ�������е����Ȼ�����Һ�����۲쵽�а�ɫ������������˵��Na2S2O3�ܱ���ˮ������
�ʴ�Ϊ��ȡ������Ӧ�����Һ�������е����Ȼ�����Һ�����۲쵽�а�ɫ������������˵��Na2S2O3�ܱ���ˮ������
�ʴ�Ϊ��=��
��2������������н�ǿ�Ļ�ԭ�ԣ�Na2S2O3��SO2�������ƣ�������������ƾ��н�ǿ�Ļ�ԭ�ԣ�
�ʴ�Ϊ����ԭ�ԣ�
��3���������ʵ��̽���ٵ�Ŀ���Dzⶨ��Һ��pH������Ϊ�ò�����պȡNa2S2O3��Һ����pH��ֽ�в�������ֽ��ɫ�����ɫ�����գ�
�ʴ�Ϊ���ò�����պȡNa2S2O3��Һ����pH��ֽ�в�������ֽ��ɫ�����ɫ�����գ�
��4���ⶨ��ҺpH=8˵����Һ�ʼ��ԣ�֤��������������ˮ���Լ��ԣ���Ӧ�����ӷ���ʽΪ��S2O32-+H2O?HS2O3-+OH-��
�ʴ�Ϊ��S2O32-+H2O?HS2O3-+OH-��
��5����������ˮ��pH��2���еμ�����Na2S2O3��Һ����ˮ����������Ʒ�Ӧ��ˮ��ɫ��dz˵������������Ӧ�����������������������Ϊ�����ƣ���Ӧ�����ӷ���ʽΪ��S2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+��
�ʴ�Ϊ��S2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+��
��6������̽�����������������ҺpH=8��֤��Na2S2O3���м��ԣ�
�ʴ�Ϊ��Na2S2O3���м��ԣ�
��7����������ˮ��pH��2���еμ�����Na2S2O3��Һ����ˮ����������Ʒ�Ӧ��ˮ��ɫ��dz˵������������Ӧ�����������������������Ϊ�����ƣ�֤��Na2S2O3���л�ԭ�ԣ�
�ʴ�Ϊ��Na2S2O3���л�ԭ�ԣ�
��8��̽��������������ˮ��pH��2���еμ�����Na2S2O3��Һ����ˮ������Һ��һ���������ӣ��������������ɳ�������֤�������������������ɵģ����Է�������ȷ��
�ʴ�Ϊ������ȷ������ˮ��������ˮ��ͬ������Cl-��
��9��֤��Na2S2O3����ˮ����������������������Ʊ������������ɵ���������Ӽ�С���飬����Ϊȡ������Ӧ�����Һ�������е����Ȼ�����Һ�����۲쵽�а�ɫ������������˵��Na2S2O3�ܱ���ˮ������
�ʴ�Ϊ��ȡ������Ӧ�����Һ�������е����Ȼ�����Һ�����۲쵽�а�ɫ������������˵��Na2S2O3�ܱ���ˮ������
���������⿼�����������ʵ�ʵ����֤������ʵ����ƣ���Ӧ�����Ӧ�ã�ʵ�鷽�����ƶϺ�ʵʩ�ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ
 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�