��Ŀ����
Ϊ�ⶨ̼��ƴ���(�躬����SiO2)��ѧ����������¼���ʵ�鷽������ش�ÿ�������е����⣮
[������](1)��ȡ̼�����ƷM g��(2)����������(3)�ռ����ⶨ���ɵ��������V mL��
���⣺���������Ʒʱ������ײ���һ��δ��ʵ���߷��ֵIJ�ȱ����ô�ⶨ��̼��ƵĴ��Ȼ�________(ƫ�ߡ�ƫ�͡���Ӱ��)
[������](1)��ȡ̼�����ƷM g��(2)��c mol/L����V mL(����)�ܽ���Ʒ��
(3)ȡ�ܽ�����Һ![]() mL����
mL����![]() mol/L��NaOH��Һ�ζ���ǡ����ȥ
mol/L��NaOH��Һ�ζ���ǡ����ȥ![]() mL��
mL��
����1���г���ʵ�������õ�����������(������̨���������ձ���������ƽ(����)��ҩ��֮��)____________________________________________��
����2����������Ƿ���Ҫ�˳�SiO2������NaOH�ζ�________(��ѡ����)��
A����Ҫ��B������Ҫ��C������
����3��̼��ƴ��ȼ��㹫ʽ______________________��
[������](1)��ȡ̼�����ƷM g��(2)����(1000��)����ֱ���������ٷ����仯����ȴ�����������Ϊ![]() g��
g��
����1��ΪʲôҪ������1000���ҡ�ֱ���������ٸı䡱��_________________________________��
����2���������еġ���ȴ��Ӧ��β���_______________________________��
[������](1)��ȡ̼�����ƷM g��(2)��������C mol/L����V mLʹ֮��ȫ�ܽ⣻(3)���˲�ȡ��Һ��(4)����Һ�м������![]() mol/L��Na2CO3��Һ
mol/L��Na2CO3��Һ![]() mL��(5)������(4)�еij����˳���ϴ�ӡ��������Ϊ
mL��(5)������(4)�еij����˳���ϴ�ӡ��������Ϊ![]() g��
g��
����1���˷����в���Ҫ��������________(��ѡ����)��
A��C��V��B��![]() ��
��![]() ��C��
��C��![]() ��D��M
��D��M
����2��Ϊ����ʵ������Ҫ�IJ�����________(��ѡ����)��
A����ȷ�ⶨNa2CO3��Һ���![]() mL
mL
B����ȷ����Ũ��![]() mol/��LNa2CO3��Һ
mol/��LNa2CO3��Һ
C��������(3)���ó���ϴ�ӣ�ϴ��ҲӦ����(4)��
D��������(3)���ó���ϴ�ӡ��������������![]() g����
g����
����3������(5)��Ҫ����������ϴ�ӣ����δ��ϴ�ӣ���ⶨ��̼���ƴ��Ƚ�________(ƫ�ߡ�ƫ�͡���Ӱ��)��
��������������Ϊ4�������У���õķ�����________������������ȱ��ֱ��ǣ�_______________
��������������ϴ�ӡ���������������̸��ӣ�������ɽϴ���
����________��____________________________________��
����________��____________________________________��
������
����(14��)������ƫ��(1��)
����������������ƽ(����)��ҩ�ס���ʽ�ͼ�ʽ�ζ��ܡ���ƿ(2��)
������B(1��)
������![]() (1��)
(1��)
���������ٱ�֤CaCO3��ȫ�ֽ�(1��)
������Ӧ�ڸ�������ȴ(1��)
��������������A��B(2��)����C(1��)����ƫ��(1��)
����������(1��)
���������������������ȷ����(1��)
����������ʵ���������ṩ1000��ĸ���(1��)

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� Ϊ�ⶨ̼��ƴ��ȣ�����������ΪSiO2����ͬѧ���������������ʵ�鷽����
Ϊ�ⶨ̼��ƴ��ȣ�����������ΪSiO2����ͬѧ���������������ʵ�鷽����
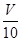 mL���Է�̪��ָʾ������c�� mol/L NaOH��Һ�ζ���ǡ����ȥV��mL��
mL���Է�̪��ָʾ������c�� mol/L NaOH��Һ�ζ���ǡ����ȥV��mL��