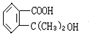��Ŀ����
ij�л���A����Է�������Ϊ60��Ϊ��һ���ⶨA�Ļ�ѧʽ����ȡ6.0 gA��ȫȼ�գ��õ�������̼��ˮ�������������Ⱥ�ͨ��������Ũ����ͼ�ʯ�ң����߷ֱ�����3.6g��8.8 g (����ÿ����Ӧ��ȫ)��
��1�����л�������ʽ�� ������ʽ�� ��
��2�����л�������̼�����Ʒ�Ӧ����������̼���ƶϸ��л���Ľṹ��ʽ�� ��
��3�����л�����̼�����Ʒ�Ӧ�����ӷ���ʽ�� ��
��4�����л��ﲻ���е������ǣ� ��
A��������Ʒ�Ӧ��B����ϡ���ᷴӦ
C������������ӦD��ʹ���Ը��������Һ��ɫ
��1��CH2O��1�֣� C2H4O2 ��1�֣� ��2�� CH3COOH��1�֣�
��3�� CH3COOH + HCO3�� =CH3COO�� + H2O + CO2��2�֣�
��4��BD��2�֣�

��ϰ��ϵ�д�
�����Ŀ