��Ŀ����
��15�֣�
ˮ������֮Դ������֮����������������Ȼ��Դ
��1���ҹ��涨ˮ�ʸ���ָ�����ҪҪ��Ϊ��a�����ó�����ɫ��b����������ζ��C��ˮӦ��������d�����ú���ϸ���Ͳ�����ǰ����ָ��������û���̿��____������ʵ�֣�������ָ�����ͨ����������������������Ӧ��������Ȼ��ͨ��_____����������ƣ���ʵ��
��2���ҹ��涨����ˮ��Ӳ�Ȳ�����25��Ӳ�ȵı�ʾ�����ǣ���ˮ�е�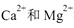 ������
������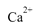 ,���������������CaO��������ͨ����1 Lˮ�к���10 mg CaO��Ϊ10��ˮ�е�
,���������������CaO��������ͨ����1 Lˮ�к���10 mg CaO��Ϊ10��ˮ�е�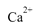 ��Mg2������һ��Ũ�ȵ�Y��Һ���еζ���Y��
��Mg2������һ��Ũ�ȵ�Y��Һ���еζ���Y��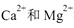 ���Ե����ʵ�����ȫ��Ӧ����ȡij�����ĵ���ˮ��Ʒ25��00 mL,��0��01000 mol/L��Y��Һ�ζ�����ȫ��Ӧʱ����Y��Һ15��00 mLa�õ���ˮ��Ӳ��Ϊ�ߣߣߣߣ��ɴ��жϸõ���ˮ�ߣߣߣ�����ϡ������ϡ�����ˮ��
���Ե����ʵ�����ȫ��Ӧ����ȡij�����ĵ���ˮ��Ʒ25��00 mL,��0��01000 mol/L��Y��Һ�ζ�����ȫ��Ӧʱ����Y��Һ15��00 mLa�õ���ˮ��Ӳ��Ϊ�ߣߣߣߣ��ɴ��жϸõ���ˮ�ߣߣߣ�����ϡ������ϡ�����ˮ��
��3��ij����ˮ��Ӳ������Ca(HC03)2������ģ����з������ܹ�ʹ֮�������ǣߣߣߣߣ�
A����ˮ������С�B������������Na3P04
C�����������ϡ���ᡡD����ˮͨ���ǻ�ú
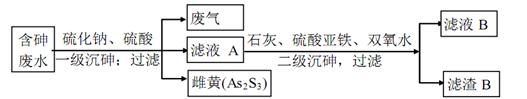
��4����ҵ�ϲ�������һʯ�����η���ȥ�����ˮ�������Ҫ������ʽΪH3ASO3����Ҫ����
��������
��֪��a��������(H3As03)��ԭ�Խ�ǿ���ױ�����Ϊ����(H3AsO3)
B���������ε��ܽ��Դ�����Ӧ��������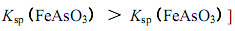
��ش��������⣺
�ٷ�������Ҫ�ɷ�Ϊ�ߣߣߣ�
��д��һ��������������ɴƻƵ����ӷ���ʽ���ߣߣߣߣߣ�
������B����Ҫ�ɷ��Уߣߣߣߣߡ�д���֣��û�ѧʽ��ʾ��
��15�֣�
��1��������2�֣� ���ˣ�2�֣�
��2��33��6º����33��6�ȣ� ��2�֣� �����ϣ�1�֣�
��3��ABD��3�֣�
��4���� H2S��1�֣�
��2H3AsO3+3S2?+6H+=As2S3��+6H2O ��2�֣�
��FeAsO4��Ca3(AsO4)2��Ca3(AsO3)2��Fe(OH)3��CaSO4��2�֣������������߾��ɣ�
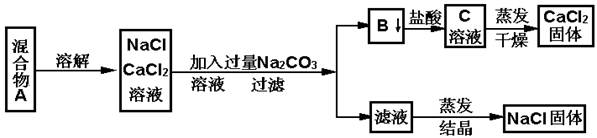
���������������1������̿���нϴ�ı���������������ԣ����ʳ�����ͨ�����˿ɳ�ȥ���ʡ�
��2��25��0����ˮ�к�Ca2+��Mg 2+�������ʵ���Ϊ0��0100mol/L��0��0150L=1��50��10-4mol���ۺϳ�CaO������Ϊ1��50��10-4��56=8��40��10-3g������ˮ��Ӳ��Ϊ8��40��10-3��1000/25��1000/10=33��6º����Ӳ�ȳ���25º���ʲ���������ˮ����
��3��A����ˮ������У�Ca(HCO3)2�ֽ�����CaCO3������Ca2+Ũ�ȼ�С����ȷ��B������������Na3P04��PO43?��Ca2+��Ӧ����Ca3(PO4)2������Ca2+Ũ�ȼ�С����ȷ��C�����������ϡ���ᣬHCl��Ca(HCO3)2��Ӧ����CaCl2��������ˮ������D����ˮͨ���ǻ�ú��Ca2+�����գ�Ca2+Ũ�ȼ�С����ȷ��
��4���ٷ�Ӧ���к������ƺ����ᣬ���Է�������Ҫ�ɷ�ΪH2S��
�ں����ˮ����H3ASO3�������Ի�������S2?������Ӧ���ɴƻ�As2S3��ͬʱ����H2O����ƽ�ɵ����ӷ���ʽ��2H3AsO3+3S2?+6H+=As2S3��+6H2O
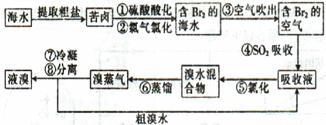
�ۼ�����ʯ�Һ�����������������Fe(OH)3��CaSO4������������ǿ������˫��ˮ������FeAsO4��Ca3(AsO4)2��Ca3(AsO3)2�ȳ�����
���㣺���⿼��ˮ�ľ�����ˮ��Ӳ�ȵļ����Ӳˮ����������ѧ���̵ķ�����

ij������Һ�к��б��ӡ����ᱽ���ã�ʵ��С��Ը÷�Һ����̽����������·�����
��֪�۵㣺����16.6�桢����43�档�е㣺����118�桢����182�档
��1��д���ڵķ�Ӧ��ѧ����ʽ ��
��2�������B�IJ��������� ��
��3���ֶ�����C�����ʽ���ʵ��̽�����������ʵ��С�鰴Ҫ�����ʵ����̼�¼���ڴ������д��ʵ�������Ԥ�������������͡�
��ѡ�Լ�������ˮ��ϡHNO3��2moL��L��1 NaOH��0.1 mol?L��1��KSCN������KMnO4��Һ��FeCl3��Һ��������ˮ����ɫʯ����Һ��
| ʵ����� | Ԥ������ | ������� |
| ����1��ȡ����C����a�Թܣ�������������ˮ���� | | |
| ���裺ȡ����C��ϡ��Һ��װb��c��֧�Թܣ���b�Թ� | ������ɫ���� | |
| ����3����c�Թ� | | C�������Լ�������ɫ��Ӧ�� |
��4����ȡһ������C��������ˮ�ܽ��ȫ��ת����1000mL����ƿ�ж��ݡ�ȡ����Һ 25.00mL������KBrO3Ũ��Ϊ0.01667 moL��L��1��KBrO3+KBr����Һ30.00mL���ữ�����á�����Ӧ��ȫ���������KI������0.1100 mol?L��1Na2S2O3����Һ�γɵ�I2����ȥNa2S2O3����Һ11.80mL����������C���ʵ����ļ������ʽΪ�� ��
�����ַ�Ӧ���ӷ���ʽ��BrO3��+5Br��+6H+=3Br2+3H2O��I2+2S2O32��=2I��+S4O62����
���к͵ζ��ķ����ⶨNaOH��Na2CO3�Ļ����Һ��NaOH�ĺ����������ڻ��Һ�м��������BaCl2��Һ��ʹNa2CO3��ȫת���BaCO3������Ȼ���ñ�����ζ�(��֪�������ָʾ����ɫ��pH��Χ���ټ���3.1��4.4 �ڼ���4.4��6.2 �۷�̪8.2��10)����ش�
��1�������BaCO3������NaOH��Һ�е������ᣬӦѡ�� ָʾ�����жϵ���ζ��յ��ʵ�������� ��
��2��Ϊ�ⶨij�ռ���Ʒ��NaOH�ĺ���(����Ʒ������ΪNa2CO3)��ijͬѧ��������ʵ�飺ȷ��ȡ5.0g��Ʒ���Ƴ�250mL��Һ��Ȼ������θ�ȡ���ƺõ��ռ���Һ20.00mL������������ˮϴ������ƿ�У��ֱ���������BaCl2��Һ��������ƿ�и�����1��2��ָʾ������Ũ��Ϊ0.2000mol��L-1�������Һ���еζ���������ݼ�¼���£�
| ʵ���� | V(�ռ���Һ)/mL | V(HCl)/mL | |
| ������ | ĩ���� | ||
| 1 | 20.00 | 0.00 | 31.00 |
| 2 | 20.00 | 1.00 | 32.04 |
| 3 | 20.00 | 1.10 | 32.18 |
�ڵζ�ʱ����ȷ������ ��
�����в����ᵼ���ռ���Ʒ��NaOH�����ⶨֵƫ�ߵ���
A����ƿ������ˮϴ��δ�ô���Һ��ϴ B����ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ
C���ڵζ�ǰ�����ݣ��ζ���������ʧ D���ζ�ǰƽ�Ӷ������ζ��������Ӷ���
14 �ֱ�����������C9H10O2������Ϊ��Ϣ���������� ����һ����ɫ��Һ�壬������ˮ������ˮ����ζ������������ˮ�㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��壬�ܼ��ȡ����Ʊ�����Ϊ�� 
��֪��
| ���� | ��Է������� | ��ɫ��״̬ | �е�(��) | �ܶ�(g��cm-3) |
| ������* | 122 | ��ɫƬ״���� | 249 | 1.2659 |
| ���������� | 150 | ��ɫ����Һ�� | 212.6 | 1.05 |
| �Ҵ� | 46 | ��ɫ����Һ�� | 78.3 | 0.7893 |
| ������ | 84 | ��ɫ����Һ�� | 80.8 | 0.7318 |
ʵ�鲽�����£�
����Բ����ƿ�м���4.0g�����ᣬ10 mL 95%���Ҵ�����������8mL �������Լ�3 mL Ũ���ᣬ��Ͼ��Ȳ������ʯ������ͼ��ʾװ�������������¶���65��70����Ȼ���2 h�����÷�ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��������������Ҵ���
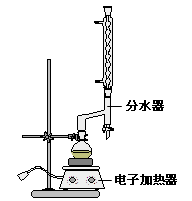
�ڷ�Ӧһ��ʱ�䣬�������ų���ˮ����Һ��ر��������������ȣ�����ˮ�����ռ�����Һ�岻���������ӣ�ֹͣ���ȡ�
�۽���ƿ�ڷ�ӦҺ����ʢ������ˮ���ձ��У���������Na2CO3����Һ�������ԡ��÷�Һ©���ֳ��л��㣬ˮ����25 mL������ȡ��Һ��Ȼ��ϲ����л��㣬�����Ȼ��ƣ����ã����ˣ�����Һ�����������������Ѻͻ�����������£�����210��213�����֡�
�ܼ���ϸ�ò�Ʒ���Ϊ2.3mL��
�ش��������⣺
��1���ڸ�ʵ���У�Բ����ƿ���ݻ����ʺϵ��� ��������ȷѡ��ǰ����ĸ����
A��25 mL B��50 mL C��100 mL D��250 mL
��2������ټ����ʯ�������� ���������һ��ʱ��������Ǽӷ�ʯ��Ӧ�ò�ȡ����ȷ�����ǣ�
��3���������ʹ�÷�ˮ�����Ϸ����ȥˮ��Ŀ����
��4������ۼ���Na2CO3���벻�㣬��֮������ʱ������ƿ�пɼ����������ɣ������������ԭ�� ��
��5���л���ķ�������У�������Ҫʹ�÷�Һ©����������ʹ�÷�Һ©��ǰ����
��6�����㱾ʵ��IJ���Ϊ ��
��������ƣ�Na2S2O3���׳Ʊ��շۣ���������Ӱ����Ҳ������ֽ��Ư�ס����ȼ��ȡ�
Na2S2O3������ˮ���������Ҵ�����������Һ����������ͨ��ΪNa2S2O��5H2O��ʵ�����Ʊ����շ۵�װ������ͼ��ʾ���漰���ܻ�ѧ����ʽ���£�

��1��������߿���װ�������Եķ����� ��
��2����Ӧǰ����a�м����ҩƷΪ ������c�м����ҩƷΪ ��
��3������b�������� ��
��4�����Ʊ������У�����ͨ������ʱ���۲쵽Bװ�������д���dz��ɫ������������Ӧһ��ʱ�䣬�������٣���dz��ɫ������ʧʱ��Ӧ��ɣ�ֹͣ���ȡ���Ӧ�����ӷ���ʽΪ ��
��5����Ӧ��ɺ�Ϊ�˴ӻ����Һ�л�ȡ��Ʒ���������£�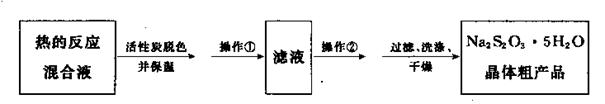
������Ϊ ��
��6��Ϊ�˲��Ʒ�Ĵ��ȣ���ȡ8.000 g�ֲ�Ʒ�����Ƴ�250 mL��Һ������Һ����ȡ25��00 mL����ƿ�У�����0.05000 mol��L��l�ĵ����Һ���еζ���2S2O32-+I2=S4O62-+2I-����
ƽ��3��ʵ�飬ƽ�����ĵ����Һ�����Ϊ30.00mL��
�ٲ�ò�Ʒ�Ĵ���Ϊ ��
�ڹ�������ʵ����йز��������±�����ȷ���� �������ţ�
| A���ü�ʽ�ζ���ʢװ�����Һ |
| B������Һ����ȡ25��00mL��Һ����ƿ�У���Һ�ܵļ������ƿ�ڱ�Ҫ�Ӵ� |
| C������ζ��յ�û�п��ƺã������Һ�μӹ�������������½��еζ� |
| D���ζ�������������ҡ����ƿ����Һ���⽦����������õĴ��Ƚ���ƫ�� |
 ��
�� ��
�� ��
�� ��]��ij��Ҫ����
��]��ij��Ҫ����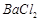 ��
��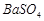 �Ļ��������ñ�����ȡ
�Ļ��������ñ�����ȡ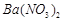 ���䲿�ֹ����������£�
���䲿�ֹ����������£�
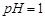 ��
��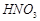 �ķ�Ӧ��ѧ����ʽΪ ��
�ķ�Ӧ��ѧ����ʽΪ ��